research use only
Nimodipine Calcium Channel inhibitor
Cat.No.S1747
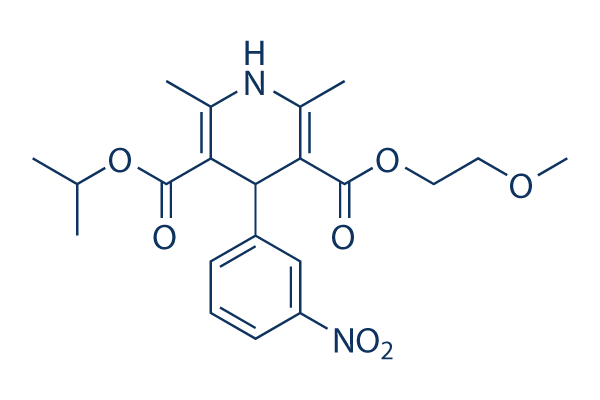
Chemical Structure
Molecular Weight: 418.44
Quality Control
| Related Targets | CFTR CRM1 CD markers AChR Sodium Channel Potassium Channel GABA Receptor TRP Channel ATPase GluR |
|---|---|
| Other Calcium Channel Inhibitors | Bay K 8644 Tetrandrine Nilvadipine Flunarizine 2HCl Cilnidipine YM-58483 (BTP2) Ionomycin Imperatorin Manidipine 2HCl Astragaloside A |
Solubility
|
In vitro |
DMSO
: 84 mg/mL
(200.74 mM)
Ethanol : 84 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 418.44 | Formula | C21H26N2O7 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 66085-59-4 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | BAY E 9736 | Smiles | CC1=C(C(C(=C(N1)C)C(=O)OC(C)C)C2=CC(=CC=C2)[N+](=O)[O-])C(=O)OCCOC | ||
Mechanism of Action
| Targets/IC50/Ki |
Calcium channel
|
|---|---|
| In vitro |
Nimodipine decreases both the peak amplitude and the integrated area of the AHP in an age- and concentration-dependent manner. This compound (100 nM) significantly reduces the AHP in aging CA1 neurons. It increases excitability in an age- and concentration-dependent manner by decreasing spike frequency accommodation (increasing the number of action potentials during prolonged depolarizing current injection). This chemical decreases accommodation only at higher concentrations in young CA1 neurons. It decreases the plateau phase of the calcium AP at concentrations as low as 100 nM in aging neurons and 10 mM in young rat neurons.
|
| In vivo |
Nimodipine results in reversible, dose-related suppression of the compound action potential of the auditory nerve (CAP; N1-P1), a prolongation of N1 latency at suprathreshold levels, an elevated CAP threshold, a decrease in N1 latency at a constant amplitude measured at CAP threshold, a reduction in cochlear microphonics (CM), and a reduction of the negative summating potential (SP) to a point where it becomes positive. This compound (10 mg/kg, SC), an L-type dihydropyridine Ca2+ channel antagonist, appears to completely block the establishment of conditioning of cocaine's effects, but only partially blocks sensitization to cocaine. This chemical (5-20 mg/kg, SC) inhibits in a dose-related manner self-administration both of cocaine and morphine contingent upon a nose-poke response.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05995405 | Recruiting | Aneurysmal Subarachnoid Hemorrhage (aSAH) |
Acasti Pharma Inc. |
October 20 2023 | Phase 3 |
| NCT04998370 | Recruiting | Subarachnoid Hemorrhage Aneurysmal|Vasospasm|Delayed Cerebral Ischemia|Delayed Ischemic Neurological Deficit |
University of Zurich |
August 18 2021 | -- |
| NCT04649398 | Recruiting | Subarachnoid Hemorrhage Aneurysmal|Delayed Cerebral Ischemia|Vasospasm Cerebral |
Medical University of Vienna|University of Vienna|Austrian Science Fund (FWF) |
November 25 2020 | -- |
| NCT02991157 | Completed | Subarachnoid Hemorrhage Aneurysmal |
National Institute of Mental Health and Neuro Sciences India |
December 2016 | -- |
| NCT01835665 | Completed | Progranulin Mutation Carriers |
University of California San Francisco|The Bluefield Project to Cure Frontotemporal Dementia |
March 2013 | Phase 1 |
| NCT01551368 | Terminated | Infertility |
Mount Sinai Hospital Canada |
December 2012 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































