- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Cilnidipine Calcium Channel inhibitor
Cat.No.S1293
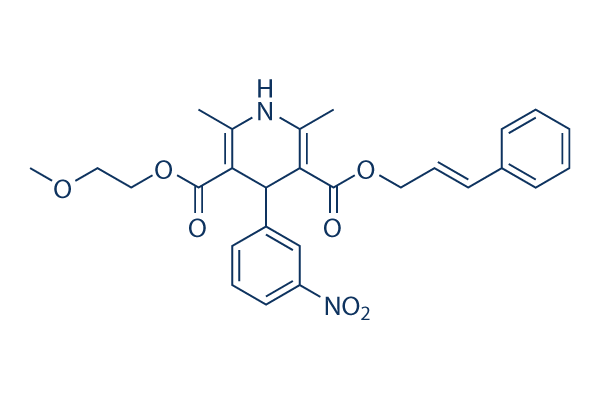
Chemical Structure
Molecular Weight: 492.52
Jump to
Quality Control
Batch:
Purity:
99.99%
99.99
| Related Targets | CFTR CRM1 CD markers AChR Sodium Channel Potassium Channel GABA Receptor TRP Channel ATPase GluR |
|---|---|
| Other Calcium Channel Inhibitors | Bay K 8644 Nilvadipine Flunarizine 2HCl YM-58483 (BTP2) Imperatorin Manidipine 2HCl Astragaloside A Benidipine HCl Azelnidipine Palmatine chloride |
Solubility
|
In vitro |
DMSO
: 99 mg/mL
(201.0 mM)
Ethanol : 15 mg/mL Water : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 492.52 | Formula | C27H28N2O7 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 132203-70-4 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | FRC-8653 | Smiles | CC1=C(C(C(=C(N1)C)C(=O)OCC=CC2=CC=CC=C2)C3=CC(=CC=C3)[N+](=O)[O-])C(=O)OCCOC | ||
Mechanism of Action
| Targets/IC50/Ki |
Calcium channel
|
|---|---|
| In vitro |
Cilnidipine (10 mM) suppresses the elevation of the ratio induced by 40 mM KCl, and this suppression is effectively inhibited after the treatment with omega-conotoxin GVIA.
|
| In vivo |
Cilnidipine reduces Ca(2+) influx via N-type Ca(2+) channels after NMDA receptors activation and then protects neurons against ischemia-reperfusion injury in the rat retina in vivo. This compound significantly prevents the increase in desmin staining and restores the glomerular podocin and nephrin expression compared with amlodipine in spontaneously hypertensive rat/ND mcr-cp (SHR/ND). It also prevents the increase in renal angiotensin II content, the expression and membrane translocation of NADPH oxidase subunits and dihydroethidium staining in SHR/ND. This chemical (30 mg/kg/d as food admix) treatment suppresses the increase in systolic blood pressure in Dahl salt-sensitive rats. It inhibits the increase in blood urea nitrogen and decrease in creatinine clearance as well as progression of glomerular sclerosis. The compound reduces plasma norepinephrine level and plasma rennin activity compared with vehicle-treated Dahl S rats. It suppresses the pressor response induced by sympathetic nerve stimulation and angiotensin II in pithed rats. This compound or omega-conotoxin MVIIA decreases mean blood pressure, but slightly increases heart rate in anesthetized rats. It can affect sympathetic N-type Ca(2+) channels in addition to vascular L-type Ca(2+) channels in antihypertensive doses in the rat in vivo.
|
References |
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































