research use only
Econazole nitrate Calcium Channel modulator
Cat.No.S2535
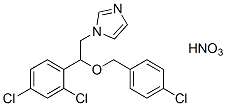
Chemical Structure
Molecular Weight: 444.7
Quality Control
| Related Targets | CFTR CRM1 CD markers AChR Sodium Channel Potassium Channel GABA Receptor TRP Channel ATPase GluR |
|---|---|
| Other Calcium Channel Inhibitors | Bay K 8644 Tetrandrine Nilvadipine Flunarizine 2HCl Cilnidipine YM-58483 (BTP2) Ionomycin Imperatorin Manidipine 2HCl Astragaloside A |
Solubility
|
In vitro |
DMSO
: 89 mg/mL
(200.13 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 444.7 | Formula | C18H15Cl3N2O.HNO3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 24169-02-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | NSC 243115,Spectazole | Smiles | C1=CC(=CC=C1COC(CN2C=CN=C2)C3=C(C=C(C=C3)Cl)Cl)Cl.[N+](=O)(O)[O-] | ||
Mechanism of Action
| Targets/IC50/Ki |
Calcium channel
|
|---|---|
| In vitro |
Econazole nitrate is an effective inducer of micronuclei over a narrow dose range in cell lines V79, XEM2 and XEMd-MZ (expresses CYP1A2). This compound inhibits the proliferation of MCF-7 cells in a time- and dose-dependent manner by MTT method and colony forming assay. This compound results in typical characteristics of apoptosis including the morphological changes and DNA fragmentation in MCF-7 cells. This compound results in the decrease expression of procaspase-3, procaspase-9 and bcl-2. This compound inhibits ADP-ribose-activated currents in HEK-293 cells expressing recombinant human TRPM2 (hTRPM2). This compound produces an essentially complete inhibition of the TRPM2-mediated current. This compound (25-50 mM) partially inhibits capacitative Ca2+ entry induced by cyclopiazonic acid, another endoplasmic reticulum Ca2+ pump inhibitor. This compound induces Ca2+ influx via two separate pathways: one is sensitive to La3+, the other is not. This compound reversibly inhibits (Bu)(2)cAMP-stimulated progesterone production in a dose- and time-dependent manner in MA-10 cells without affecting total protein synthesis or P450(scc) and 3beta-hydroxysteroid dehydrogenase (3beta-HSD) enzyme expression or activity. This compound is a store-operated Ca2+ channel antagonist which induces cytotoxic cell death of leukemia. This compound (5-20 mM) arrests human colon cancer cells at the G0/G1 phase of the cell cycle. This compound induces COLO 205 cells apoptosis evidenced by ladder formation in DNA fragmentation assay and sub-G1 peak. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT01696799 | Completed | Interdigital Tinea Pedis |
AmDerma|AmDerma Pharmaceuticals LLC |
September 2011 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































