research use only
Vinorelbine ditartrate Microtubule Associated inhibitor
Cat.No.S4269
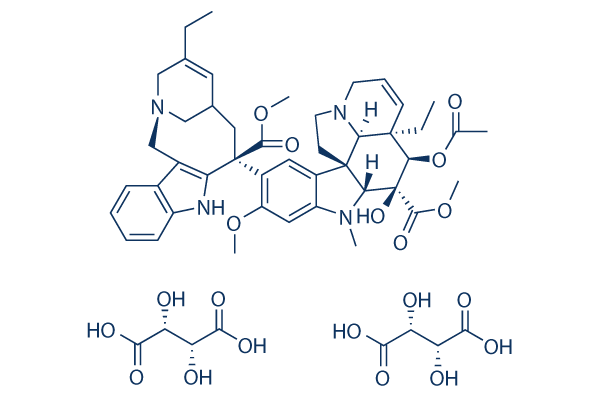
Chemical Structure
Molecular Weight: 1079.11
Quality Control
| Related Targets | Akt Wnt/beta-catenin PKC HSP ROCK Integrin Bcr-Abl Actin FAK Kinesin |
|---|---|
| Other Microtubule Associated Inhibitors | Nocodazole MMAF Patupilone (Epothilone B) CW069 Lexibulin (CYT997) Combretastatin A4 Epothilone A ABT-751 (E7010) Cucurbitacin B TAI-1 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| JEG3 | Cell viability assay | 0.01-100 μM | 48 h | vinorelbine significantly reduced JEG3 cell viability in a dose-dependent manner, down to a concentration of 0.1 μM. | 29429891 | |
| HTR-8/SVneo | Cell viability assay | 0.01-100 μM | 48 h | vinorelbine significantly reduced HTR-8/svneo cell viability in a dose-dependent manner, down to a concentration of 1 μM. | 29429891 | |
| EBC-1 | Function assay | EBC-1 cells displayed a markedly higher rate of cell death upon capmatinib treatment, albeit not reaching 100%, whereas the effect in NCI-H1993 was largely restricted to inhibition of proliferation | 30674502 | |||
| NCI-H1993 | Function assay | the rate of cell death upon capmatinib treatment was largely restricted to inhibition of proliferation | 30674502 | |||
| SCC114 | Antiproliferative assay | 72 hrs | Antiproliferative activity against exponentially growing adherent human SCC114 cells assessed as inhibition of cell proliferation after 72 hrs by luminescence detection based ATPlite assay, IC50 = 0.00007 μM. | 25872984 | ||
| KB | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB cells after 72 hrs, IC50 = 0.001 μM. | 19072542 | ||
| U87MG | Cytotoxicity assay | 72 hrs | Cytotoxicity against human U87MG cells after 72 hrs by resazurin assay, IC50 = 0.002 μM. | 23822556 | ||
| U87 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human U87 cells after 72 hrs, IC50 = 0.002 μM. | 24871162 | ||
| MCF7 | Cytotoxicity assay | Cytotoxicity against human MCF7 cells, GI50 = 0.0024 μM. | 19147348 | |||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells, GI50 = 0.0027 μM. | 19147348 | |||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells incubated for 72 hrs by CellTiter 96 aqueous one solution reagent based assay, IC50 = 0.003 μM. | 27753480 | ||
| K562 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human K562 cells after 72 hrs, IC50 = 0.0035 μM. | 24871162 | ||
| U87 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human U87 cells assessed as inhibition of cell proliferation after 72 hrs, IC50 = 0.0035 μM. | 25804719 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells after 72 hrs, IC50 = 0.004 μM. | 24871162 | ||
| KB | Cell cycle assay | 24 hrs | Cell cycle arrest in human KB cells assessed as accumulation of cells at G2/M phase after 24 hrs by propidium iodide-based FACScan, EC50 = 0.006 μM. | 21480626 | ||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells assessed as inhibition of cell proliferation after 72 hrs, IC50 = 0.006 μM. | 25804719 | ||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs by resazurin-based fluorimetric assay, IC50 = 0.006 μM. | 24901800 | ||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells by MTT assay, IC50 = 0.009 μM. | 22525316 | |||
| A549 | Cytotoxicity assay | 24 to 72 hrs | Cytotoxicity against human A549 cells after 24 to 72 hrs by MTT assay, IC50 = 0.009 μM. | 23107481 | ||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by MTT assay, IC50 = 0.01 μM. | 22525316 | |||
| HT-29 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HT-29 cells after 72 hrs by sulforhodamine B assay, IC50 = 0.0119 μM. | 21480626 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by resazurin-based fluorimetric assay, IC50 = 0.015 μM. | 24901800 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells assessed as inhibition of cell proliferation after 72 hrs, IC50 = 0.015 μM. | 25804719 | ||
| K562 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human K562 cells after 72 hrs by resazurin assay, IC50 = 0.02 μM. | 23822556 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells after 72 hrs by SRB assay, IC50 = 0.026 μM. | 17544278 | ||
| SKOV3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SKOV3 cells after 72 hrs by sulforhodamine B assay, IC50 = 0.0267 μM. | 21480626 | ||
| HeLa | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HeLa cells after 72 hrs by SRB assay, IC50 = 0.0269 μM. | 18771244 | ||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells by sulforhodamine B assay, IC50 = 0.027 μM. | 19499938 | |||
| HeLa | Cytotoxicity assay | Cytotoxicity against human HeLa cells by MTT assay, IC50 = 0.027 μM. | 22115594 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by sulforhodamine B assay, IC50 = 0.03 μM. | 19499938 | |||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by resazurin assay, IC50 = 0.035 μM. | 23822556 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells after 72 hrs, IC50 = 0.035 μM. | 24871162 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by SRB assay, IC50 = 0.0369 μM. | 18771244 | ||
| KB | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB cells after 72 hrs, IC50 = 0.04 μM. | 19072542 | ||
| NCI-H460 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human NCI-H460 cells after 72 hrs by sulforhodamine B assay, IC50 = 0.0437 μM. | 21480626 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by SRB assay, IC50 = 0.049 μM. | 17544278 | ||
| HL60 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HL60 cells after 48 hrs by MTT assay, IC50 = 0.06 μM. | 20462230 | ||
| A431 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A431 cells after 72 hrs by sulforhodamine B assay, IC50 = 0.06 μM. | 21480626 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells after 72 hrs by sulforhodamine B assay, IC50 = 0.0646 μM. | 21480626 | ||
| L1210 | Cytotoxicity assay | 72 hrs | Cytotoxicity against mouse L1210 cells assessed as growth inhibition after 72 hrs by phosphatase assay, IC50 = 0.065 μM. | 23252481 | ||
| HeLa | Function assay | 4.5 hrs | Inhibition of Ebolavirus glycoprotein/matrix protein VP40 entry in human HeLa cells after 4.5 hrs beta-lactamase reporter assay, IC50 = 0.066 μM. | 29624387 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, IC50 = 0.07 μM. | 19743863 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells assessed as growth inhibition after 72 hrs by phosphatase assay, IC50 = 0.075 μM. | 23252481 | ||
| OVCAR8 | Growth inhibition assay | 96 hrs | Growth inhibition of human OVCAR8 cells overexpressing P-glycoprotein after 96 hrs, IC50 = 0.3 μM. | 22044164 | ||
| OVCAR8 | Cytotoxicity assay | Cytotoxicity against human OVCAR8 cells assessed as growth inhibition by trypan blue exclusion assay, IC50 = 0.3 μM. | 23214452 | |||
| OVCAR8 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human OVCAR8 cells assessed as growth inhibition after 96 hrs by Giemsa staining-based light microscopy, IC50 = 0.3 μM. | 25025991 | ||
| OVCAR8 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human OVCAR8 cells assessed as growth inhibition after 48 hrs by SRB assay, IC50 = 0.3 μM. | 26132075 | ||
| OVCAR8 | Growth inhibition assay | 96 hrs | Growth inhibition of human OVCAR8 cells after 96 hrs by trypan blue exclusion assay, IC50 = 0.3 μM. | 29730191 | ||
| K562R | Cytotoxicity assay | 72 hrs | Cytotoxicity against doxorubicin-resistant human K562R cells incubated for 72 hrs by CellTiter 96 aqueous one solution reagent based assay, IC50 = 0.41 μM. | 27753480 | ||
| HCT116/VM46 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human vinblastine-resistant HCT116/VM46 cells assessed as growth inhibition after 72 hrs by phosphatase assay, IC50 = 0.6 μM. | 23252481 | ||
| SMMC7721 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human SMMC7721 cells after 48 hrs by MTT assay, IC50 = 4.7 μM. | 20462230 | ||
| NCI-ADR-RES | Growth inhibition assay | 96 hrs | Growth inhibition of doxorubicin-resistant human NCI-ADR-RES cells overexpressing P-glycoprotein after 96 hrs, IC50 = 5 μM. | 22044164 | ||
| NCI/ADR-RES | Cytotoxicity assay | Cytotoxicity against human NCI/ADR-RES cells assessed as growth inhibition by trypan blue exclusion assay, IC50 = 5 μM. | 23214452 | |||
| NCI/ADR-RES | Cytotoxicity assay | 96 hrs | Cytotoxicity against human NCI/ADR-RES cells assessed as growth inhibition after 96 hrs by Giemsa staining-based light microscopy, IC50 = 5 μM. | 25025991 | ||
| NCI-ADR-RES | Cytotoxicity assay | 48 hrs | Cytotoxicity against Pgp overexpressing human NCI-ADR-RES cells assessed as growth inhibition after 48 hrs by SRB assay, IC50 = 5 μM. | 26132075 | ||
| NCI/ADR-RES | Growth inhibition assay | 96 hrs | Growth inhibition of human NCI/ADR-RES cells after 96 hrs by trypan blue exclusion assay, IC50 = 5 μM. | 29730191 | ||
| BESM | Antitrypanosomal assay | 88 hrs | Antitrypanosomal activity against Trypanosoma cruzi amastigotes infected in BESM cells measured after 88 hrs postinfection by HTS assay, EC50 = 10 μM. | 20547819 | ||
| HL60 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HL60 cells after 48 hrs by MTT assay, IC50 = 11.47 μM. | 26420067 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50 = 17.2 μM. | 20462230 | ||
| HepG2 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50 = 23.12 μM. | 26420067 | ||
| A549 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human A549 cells after 48 hrs by MTT assay, IC50 = 26.1 μM. | 20462230 | ||
| U2OS | Apoptosis assay | 72 hrs | Induction of apoptosis in human U2OS cells assessed as increase in caspase-3 catalytic activity at cytotoxic IC50 level after 72 hrs | 25768699 | ||
| U2OS | Function assay | 72 hrs | Inhibition of tubulin polymerization in human U2OS cells at cytotoxic IC50 level after 72 hrs by Western blotting method | 25768699 | ||
| STO | Function assay | 2 nM | 24 hrs | Inhibition of alpha-tubulin polymerization in human human STO cells assessed as increase in ratio of soluble to polymerized tubulin fraction at 2 nM after 24 hrs by western blot analysis | 27643641 | |
| MP8 | Function assay | 13 nM | 24 hrs | Inhibition of alpha-tubulin polymerization in human human MP8 cells assessed as increase in ratio of soluble to polymerized tubulin fraction at 13 nM after 24 hrs by western blot analysis | 27643641 | |
| STO | Cell cycle assay | 48 to 72 hrs | Cell cycle arrest in human STO cells assessed as accumulation at G2/M phase at antiproliferative IC50 after 48 to 72 hrs by propidium iodide staining based flow cytometry | 27643641 | ||
| MP8 | Cell cycle assay | 48 to 72 hrs | Cell cycle arrest in human MP8 cells assessed as accumulation at G2/M phase at antiproliferative IC50 after 48 to 72 hrs by propidium iodide staining based flow cytometry | 27643641 | ||
| COLO-829 | Growth inhibition assay | Inhibition of human COLO-829 cell growth in a cell viability assay, IC50 = 0.0001051 μM. | SANGER | |||
| CGTH-W-1 | Growth inhibition assay | Inhibition of human CGTH-W-1 cell growth in a cell viability assay, IC50 = 0.0002316 μM. | SANGER | |||
| BB30-HNC | Growth inhibition assay | Inhibition of human BB30-HNC cell growth in a cell viability assay, IC50 = 0.0003988 μM. | SANGER | |||
| ECC12 | Growth inhibition assay | Inhibition of human ECC12 cell growth in a cell viability assay, IC50 = 0.000509 μM. | SANGER | |||
| NCI-H522 | Growth inhibition assay | Inhibition of human NCI-H522 cell growth in a cell viability assay, IC50 = 0.0005354 μM. | SANGER | |||
| HT-144 | Growth inhibition assay | Inhibition of human HT-144 cell growth in a cell viability assay, IC50 = 0.0005909 μM. | SANGER | |||
| GT3TKB | Growth inhibition assay | Inhibition of human GT3TKB cell growth in a cell viability assay, IC50 = 0.0007477 μM. | SANGER | |||
| NTERA-S-cl-D1 | Growth inhibition assay | Inhibition of human NTERA-S-cl-D1 cell growth in a cell viability assay, IC50 = 0.0009386 μM. | SANGER | |||
| TE-9 | Growth inhibition assay | Inhibition of human TE-9 cell growth in a cell viability assay, IC50 = 0.001078 μM. | SANGER | |||
| A253 | Growth inhibition assay | Inhibition of human A253 cell growth in a cell viability assay, IC50 = 0.001223 μM. | SANGER | |||
| ACN | Growth inhibition assay | Inhibition of human ACN cell growth in a cell viability assay, IC50 = 0.001329 μM. | SANGER | |||
| COLO-205 | Growth inhibition assay | Inhibition of human COLO-205 cell growth in a cell viability assay, IC50 = 0.001368 μM. | SANGER | |||
| LCLC-103H | Growth inhibition assay | Inhibition of human LCLC-103H cell growth in a cell viability assay, IC50 = 0.001427 μM. | SANGER | |||
| SK-LMS-1 | Growth inhibition assay | Inhibition of human SK-LMS-1 cell growth in a cell viability assay, IC50 = 0.001461 μM. | SANGER | |||
| KS-1 | Growth inhibition assay | Inhibition of human KS-1 cell growth in a cell viability assay, IC50 = 0.001466 μM. | SANGER | |||
| MC-IXC | Growth inhibition assay | Inhibition of human MC-IXC cell growth in a cell viability assay, IC50 = 0.001486 μM. | SANGER | |||
| RKO | Growth inhibition assay | Inhibition of human RKO cell growth in a cell viability assay, IC50 = 0.00152 μM. | SANGER | |||
| LCLC-97TM1 | Growth inhibition assay | Inhibition of human LCLC-97TM1 cell growth in a cell viability assay, IC50 = 0.001559 μM. | SANGER | |||
| OVCAR-4 | Growth inhibition assay | Inhibition of human OVCAR-4 cell growth in a cell viability assay, IC50 = 0.001745 μM. | SANGER | |||
| COLO-679 | Growth inhibition assay | Inhibition of human COLO-679 cell growth in a cell viability assay, IC50 = 0.001832 μM. | SANGER | |||
| HGC-27 | Growth inhibition assay | Inhibition of human HGC-27 cell growth in a cell viability assay, IC50 = 0.001852 μM. | SANGER | |||
| NCI-H520 | Growth inhibition assay | Inhibition of human NCI-H520 cell growth in a cell viability assay, IC50 = 0.00187 μM. | SANGER | |||
| BPH-1 | Growth inhibition assay | Inhibition of human BPH-1 cell growth in a cell viability assay, IC50 = 0.001899 μM. | SANGER | |||
| IGR-1 | Growth inhibition assay | Inhibition of human IGR-1 cell growth in a cell viability assay, IC50 = 0.001903 μM. | SANGER | |||
| HOP-62 | Growth inhibition assay | Inhibition of human HOP-62 cell growth in a cell viability assay, IC50 = 0.001927 μM. | SANGER | |||
| ATN-1 | Growth inhibition assay | Inhibition of human ATN-1 cell growth in a cell viability assay, IC50 = 0.001943 μM. | SANGER | |||
| SCC-9 | Growth inhibition assay | Inhibition of human SCC-9 cell growth in a cell viability assay, IC50 = 0.002046 μM. | SANGER | |||
| SBC-5 | Growth inhibition assay | Inhibition of human SBC-5 cell growth in a cell viability assay, IC50 = 0.002082 μM. | SANGER | |||
| NB12 | Growth inhibition assay | Inhibition of human NB12 cell growth in a cell viability assay, IC50 = 0.002111 μM. | SANGER | |||
| HUTU-80 | Growth inhibition assay | Inhibition of human HUTU-80 cell growth in a cell viability assay, IC50 = 0.002177 μM. | SANGER | |||
| IGROV-1 | Growth inhibition assay | Inhibition of human IGROV-1 cell growth in a cell viability assay, IC50 = 0.002202 μM. | SANGER | |||
| SK-UT-1 | Growth inhibition assay | Inhibition of human SK-UT-1 cell growth in a cell viability assay, IC50 = 0.002234 μM. | SANGER | |||
| HSC-2 | Growth inhibition assay | Inhibition of human HSC-2 cell growth in a cell viability assay, IC50 = 0.00225 μM. | SANGER | |||
| MKN45 | Growth inhibition assay | Inhibition of human MKN45 cell growth in a cell viability assay, IC50 = 0.002287 μM. | SANGER | |||
| SW1710 | Growth inhibition assay | Inhibition of human SW1710 cell growth in a cell viability assay, IC50 = 0.002334 μM. | SANGER | |||
| SIG-M5 | Growth inhibition assay | Inhibition of human SIG-M5 cell growth in a cell viability assay, IC50 = 0.002476 μM. | SANGER | |||
| HT-1080 | Growth inhibition assay | Inhibition of human HT-1080 cell growth in a cell viability assay, IC50 = 0.002495 μM. | SANGER | |||
| SCC-4 | Growth inhibition assay | Inhibition of human SCC-4 cell growth in a cell viability assay, IC50 = 0.002585 μM. | SANGER | |||
| NCI-H2122 | Growth inhibition assay | Inhibition of human NCI-H2122 cell growth in a cell viability assay, IC50 = 0.002588 μM. | SANGER | |||
| KM12 | Growth inhibition assay | Inhibition of human KM12 cell growth in a cell viability assay, IC50 = 0.002606 μM. | SANGER | |||
| DMS-273 | Growth inhibition assay | Inhibition of human DMS-273 cell growth in a cell viability assay, IC50 = 0.002707 μM. | SANGER | |||
| KP-4 | Growth inhibition assay | Inhibition of human KP-4 cell growth in a cell viability assay, IC50 = 0.002711 μM. | SANGER | |||
| ES8 | Growth inhibition assay | Inhibition of human ES8 cell growth in a cell viability assay, IC50 = 0.002719 μM. | SANGER | |||
| HuCCT1 | Growth inhibition assay | Inhibition of human HuCCT1 cell growth in a cell viability assay, IC50 = 0.002778 μM. | SANGER | |||
| KURAMOCHI | Growth inhibition assay | Inhibition of human KURAMOCHI cell growth in a cell viability assay, IC50 = 0.002814 μM. | SANGER | |||
| NCI-H1703 | Growth inhibition assay | Inhibition of human NCI-H1703 cell growth in a cell viability assay, IC50 = 0.002834 μM. | SANGER | |||
| A431 | Growth inhibition assay | Inhibition of human A431 cell growth in a cell viability assay, IC50 = 0.002897 μM. | SANGER | |||
| NCI-H1355 | Growth inhibition assay | Inhibition of human NCI-H1355 cell growth in a cell viability assay, IC50 = 0.0029 μM. | SANGER | |||
| MG-63 | Growth inhibition assay | Inhibition of human MG-63 cell growth in a cell viability assay, IC50 = 0.002938 μM. | SANGER | |||
| ST486 | Growth inhibition assay | Inhibition of human ST486 cell growth in a cell viability assay, IC50 = 0.002957 μM. | SANGER | |||
| BCPAP | Growth inhibition assay | Inhibition of human BCPAP cell growth in a cell viability assay, IC50 = 0.002978 μM. | SANGER | |||
| 786-0 | Growth inhibition assay | Inhibition of human 786-0 cell growth in a cell viability assay, IC50 = 0.002992 μM. | SANGER | |||
| ABC-1 | Growth inhibition assay | Inhibition of human ABC-1 cell growth in a cell viability assay, IC50 = 0.003024 μM. | SANGER | |||
| KYSE-150 | Growth inhibition assay | Inhibition of human KYSE-150 cell growth in a cell viability assay, IC50 = 0.003039 μM. | SANGER | |||
| QIMR-WIL | Growth inhibition assay | Inhibition of human QIMR-WIL cell growth in a cell viability assay, IC50 = 0.003043 μM. | SANGER | |||
| A427 | Growth inhibition assay | Inhibition of human A427 cell growth in a cell viability assay, IC50 = 0.003103 μM. | SANGER | |||
| TE-15 | Growth inhibition assay | Inhibition of human TE-15 cell growth in a cell viability assay, IC50 = 0.003103 μM. | SANGER | |||
| MES-SA | Growth inhibition assay | Inhibition of human MES-SA cell growth in a cell viability assay, IC50 = 0.003115 μM. | SANGER | |||
| PA-1 | Growth inhibition assay | Inhibition of human PA-1 cell growth in a cell viability assay, IC50 = 0.003123 μM. | SANGER | |||
| KYSE-270 | Growth inhibition assay | Inhibition of human KYSE-270 cell growth in a cell viability assay, IC50 = 0.003156 μM. | SANGER | |||
| BL-70 | Growth inhibition assay | Inhibition of human BL-70 cell growth in a cell viability assay, IC50 = 0.003164 μM. | SANGER | |||
| LS-411N | Growth inhibition assay | Inhibition of human LS-411N cell growth in a cell viability assay, IC50 = 0.003171 μM. | SANGER | |||
| A204 | Growth inhibition assay | Inhibition of human A204 cell growth in a cell viability assay, IC50 = 0.003208 μM. | SANGER | |||
| HOS | Growth inhibition assay | Inhibition of human HOS cell growth in a cell viability assay, IC50 = 0.003218 μM. | SANGER | |||
| HT-29 | Growth inhibition assay | Inhibition of human HT-29 cell growth in a cell viability assay, IC50 = 0.003236 μM. | SANGER | |||
| PC-14 | Growth inhibition assay | Inhibition of human PC-14 cell growth in a cell viability assay, IC50 = 0.00326 μM. | SANGER | |||
| MEL-HO | Growth inhibition assay | Inhibition of human MEL-HO cell growth in a cell viability assay, IC50 = 0.003291 μM. | SANGER | |||
| ONS-76 | Growth inhibition assay | Inhibition of human ONS-76 cell growth in a cell viability assay, IC50 = 0.0033 μM. | SANGER | |||
| ALL-PO | Growth inhibition assay | Inhibition of human ALL-PO cell growth in a cell viability assay, IC50 = 0.003359 μM. | SANGER | |||
| RT-112 | Growth inhibition assay | Inhibition of human RT-112 cell growth in a cell viability assay, IC50 = 0.003371 μM. | SANGER | |||
| HCT-116 | Growth inhibition assay | Inhibition of human HCT-116 cell growth in a cell viability assay, IC50 = 0.003373 μM. | SANGER | |||
| 8-MG-BA | Growth inhibition assay | Inhibition of human 8-MG-BA cell growth in a cell viability assay, IC50 = 0.003375 μM. | SANGER | |||
| A2058 | Growth inhibition assay | Inhibition of human A2058 cell growth in a cell viability assay, IC50 = 0.003503 μM. | SANGER | |||
| PANC-10-05 | Growth inhibition assay | Inhibition of human PANC-10-05 cell growth in a cell viability assay, IC50 = 0.003525 μM. | SANGER | |||
| G-361 | Growth inhibition assay | Inhibition of human G-361 cell growth in a cell viability assay, IC50 = 0.003525 μM. | SANGER | |||
| GI-1 | Growth inhibition assay | Inhibition of human GI-1 cell growth in a cell viability assay, IC50 = 0.003536 μM. | SANGER | |||
| DoTc2-4510 | Growth inhibition assay | Inhibition of human DoTc2-4510 cell growth in a cell viability assay, IC50 = 0.003593 μM. | SANGER | |||
| NCI-H2030 | Growth inhibition assay | Inhibition of human NCI-H2030 cell growth in a cell viability assay, IC50 = 0.003618 μM. | SANGER | |||
| BFTC-905 | Growth inhibition assay | Inhibition of human BFTC-905 cell growth in a cell viability assay, IC50 = 0.003622 μM. | SANGER | |||
| TE-10 | Growth inhibition assay | Inhibition of human TE-10 cell growth in a cell viability assay, IC50 = 0.003625 μM. | SANGER | |||
| DEL | Growth inhibition assay | Inhibition of human DEL cell growth in a cell viability assay, IC50 = 0.00363 μM. | SANGER | |||
| IA-LM | Growth inhibition assay | Inhibition of human IA-LM cell growth in a cell viability assay, IC50 = 0.003713 μM. | SANGER | |||
| YKG-1 | Growth inhibition assay | Inhibition of human YKG-1 cell growth in a cell viability assay, IC50 = 0.00373 μM. | SANGER | |||
| ES1 | Growth inhibition assay | Inhibition of human ES1 cell growth in a cell viability assay, IC50 = 0.003778 μM. | SANGER | |||
| ES7 | Growth inhibition assay | Inhibition of human ES7 cell growth in a cell viability assay, IC50 = 0.003856 μM. | SANGER | |||
| RVH-421 | Growth inhibition assay | Inhibition of human RVH-421 cell growth in a cell viability assay, IC50 = 0.003881 μM. | SANGER | |||
| SNG-M | Growth inhibition assay | Inhibition of human SNG-M cell growth in a cell viability assay, IC50 = 0.003883 μM. | SANGER | |||
| DBTRG-05MG | Growth inhibition assay | Inhibition of human DBTRG-05MG cell growth in a cell viability assay, IC50 = 0.003885 μM. | SANGER | |||
| HC-1 | Growth inhibition assay | Inhibition of human HC-1 cell growth in a cell viability assay, IC50 = 0.003887 μM. | SANGER | |||
| MHH-ES-1 | Growth inhibition assay | Inhibition of human MHH-ES-1 cell growth in a cell viability assay, IC50 = 0.003894 μM. | SANGER | |||
| KALS-1 | Growth inhibition assay | Inhibition of human KALS-1 cell growth in a cell viability assay, IC50 = 0.003897 μM. | SANGER | |||
| BxPC-3 | Growth inhibition assay | Inhibition of human BxPC-3 cell growth in a cell viability assay, IC50 = 0.003901 μM. | SANGER | |||
| NCI-H810 | Growth inhibition assay | Inhibition of human NCI-H810 cell growth in a cell viability assay, IC50 = 0.003908 μM. | SANGER | |||
| NCI-H1648 | Growth inhibition assay | Inhibition of human NCI-H1648 cell growth in a cell viability assay, IC50 = 0.00392 μM. | SANGER | |||
| EW-1 | Growth inhibition assay | Inhibition of human EW-1 cell growth in a cell viability assay, IC50 = 0.003934 μM. | SANGER | |||
| A4-Fuk | Growth inhibition assay | Inhibition of human A4-Fuk cell growth in a cell viability assay, IC50 = 0.004035 μM. | SANGER | |||
| KYSE-510 | Growth inhibition assay | Inhibition of human KYSE-510 cell growth in a cell viability assay, IC50 = 0.004188 μM. | SANGER | |||
| 697 | Growth inhibition assay | Inhibition of human 697 cell growth in a cell viability assay, IC50 = 0.004228 μM. | SANGER | |||
| VA-ES-BJ | Growth inhibition assay | Inhibition of human VA-ES-BJ cell growth in a cell viability assay, IC50 = 0.004233 μM. | SANGER | |||
| MEL-JUSO | Growth inhibition assay | Inhibition of human MEL-JUSO cell growth in a cell viability assay, IC50 = 0.004255 μM. | SANGER | |||
| CAL-39 | Growth inhibition assay | Inhibition of human CAL-39 cell growth in a cell viability assay, IC50 = 0.004258 μM. | SANGER | |||
| ML-2 | Growth inhibition assay | Inhibition of human ML-2 cell growth in a cell viability assay, IC50 = 0.004323 μM. | SANGER | |||
| EW-16 | Growth inhibition assay | Inhibition of human EW-16 cell growth in a cell viability assay, IC50 = 0.004348 μM. | SANGER | |||
| 8505C | Growth inhibition assay | Inhibition of human 8505C cell growth in a cell viability assay, IC50 = 0.004419 μM. | SANGER | |||
| KE-37 | Growth inhibition assay | Inhibition of human KE-37 cell growth in a cell viability assay, IC50 = 0.00442 μM. | SANGER | |||
| OCUB-M | Growth inhibition assay | Inhibition of human OCUB-M cell growth in a cell viability assay, IC50 = 0.004433 μM. | SANGER | |||
| Ramos-2G6-4C10 | Growth inhibition assay | Inhibition of human Ramos-2G6-4C10 cell growth in a cell viability assay, IC50 = 0.004452 μM. | SANGER | |||
| MRK-nu-1 | Growth inhibition assay | Inhibition of human MRK-nu-1 cell growth in a cell viability assay, IC50 = 0.004474 μM. | SANGER | |||
| SK-HEP-1 | Growth inhibition assay | Inhibition of human SK-HEP-1 cell growth in a cell viability assay, IC50 = 0.004517 μM. | SANGER | |||
| CCRF-CEM | Growth inhibition assay | Inhibition of human CCRF-CEM cell growth in a cell viability assay, IC50 = 0.004555 μM. | SANGER | |||
| CHL-1 | Growth inhibition assay | Inhibition of human CHL-1 cell growth in a cell viability assay, IC50 = 0.004595 μM. | SANGER | |||
| LXF-289 | Growth inhibition assay | Inhibition of human LXF-289 cell growth in a cell viability assay, IC50 = 0.004685 μM. | SANGER | |||
| SW13 | Growth inhibition assay | Inhibition of human SW13 cell growth in a cell viability assay, IC50 = 0.004717 μM. | SANGER | |||
| SUP-T1 | Growth inhibition assay | Inhibition of human SUP-T1 cell growth in a cell viability assay, IC50 = 0.004719 μM. | SANGER | |||
| HMV-II | Growth inhibition assay | Inhibition of human HMV-II cell growth in a cell viability assay, IC50 = 0.004768 μM. | SANGER | |||
| MKN28 | Growth inhibition assay | Inhibition of human MKN28 cell growth in a cell viability assay, IC50 = 0.004771 μM. | SANGER | |||
| NCI-H2342 | Growth inhibition assay | Inhibition of human NCI-H2342 cell growth in a cell viability assay, IC50 = 0.004785 μM. | SANGER | |||
| DU-145 | Growth inhibition assay | Inhibition of human DU-145 cell growth in a cell viability assay, IC50 = 0.004814 μM. | SANGER | |||
| DSH1 | Growth inhibition assay | Inhibition of human DSH1 cell growth in a cell viability assay, IC50 = 0.004868 μM. | SANGER | |||
| HCC38 | Growth inhibition assay | Inhibition of human HCC38 cell growth in a cell viability assay, IC50 = 0.004872 μM. | SANGER | |||
| CAL-85-1 | Growth inhibition assay | Inhibition of human CAL-85-1 cell growth in a cell viability assay, IC50 = 0.004904 μM. | SANGER | |||
| 8305C | Growth inhibition assay | Inhibition of human 8305C cell growth in a cell viability assay, IC50 = 0.004919 μM. | SANGER | |||
| HCC1569 | Growth inhibition assay | Inhibition of human HCC1569 cell growth in a cell viability assay, IC50 = 0.004932 μM. | SANGER | |||
| NKM-1 | Growth inhibition assay | Inhibition of human NKM-1 cell growth in a cell viability assay, IC50 = 0.004972 μM. | SANGER | |||
| LC-2-ad | Growth inhibition assay | Inhibition of human LC-2-ad cell growth in a cell viability assay, IC50 = 0.004977 μM. | SANGER | |||
| CTV-1 | Growth inhibition assay | Inhibition of human CTV-1 cell growth in a cell viability assay, IC50 = 0.005011 μM. | SANGER | |||
| MCF7 | Growth inhibition assay | Inhibition of human MCF7 cell growth in a cell viability assay, IC50 = 0.005011 μM. | SANGER | |||
| NB13 | Growth inhibition assay | Inhibition of human NB13 cell growth in a cell viability assay, IC50 = 0.005014 μM. | SANGER | |||
| CAL-33 | Growth inhibition assay | Inhibition of human CAL-33 cell growth in a cell viability assay, IC50 = 0.005021 μM. | SANGER | |||
| DOHH-2 | Growth inhibition assay | Inhibition of human DOHH-2 cell growth in a cell viability assay, IC50 = 0.005039 μM. | SANGER | |||
| CESS | Growth inhibition assay | Inhibition of human CESS cell growth in a cell viability assay, IC50 = 0.005043 μM. | SANGER | |||
| HCC2998 | Growth inhibition assay | Inhibition of human HCC2998 cell growth in a cell viability assay, IC50 = 0.005045 μM. | SANGER | |||
| NCI-H292 | Growth inhibition assay | Inhibition of human NCI-H292 cell growth in a cell viability assay, IC50 = 0.005053 μM. | SANGER | |||
| RXF393 | Growth inhibition assay | Inhibition of human RXF393 cell growth in a cell viability assay, IC50 = 0.005065 μM. | SANGER | |||
| BL-41 | Growth inhibition assay | Inhibition of human BL-41 cell growth in a cell viability assay, IC50 = 0.00528 μM. | SANGER | |||
| SW982 | Growth inhibition assay | Inhibition of human SW982 cell growth in a cell viability assay, IC50 = 0.005298 μM. | SANGER | |||
| Becker | Growth inhibition assay | Inhibition of human Becker cell growth in a cell viability assay, IC50 = 0.005391 μM. | SANGER | |||
| NCCIT | Growth inhibition assay | Inhibition of human NCCIT cell growth in a cell viability assay, IC50 = 0.005419 μM. | SANGER | |||
| RD | Growth inhibition assay | Inhibition of human RD cell growth in a cell viability assay, IC50 = 0.005425 μM. | SANGER | |||
| NCI-H1770 | Growth inhibition assay | Inhibition of human NCI-H1770 cell growth in a cell viability assay, IC50 = 0.005534 μM. | SANGER | |||
| SNU-423 | Growth inhibition assay | Inhibition of human SNU-423 cell growth in a cell viability assay, IC50 = 0.00554 μM. | SANGER | |||
| MFH-ino | Growth inhibition assay | Inhibition of human MFH-ino cell growth in a cell viability assay, IC50 = 0.005596 μM. | SANGER | |||
| HSC-3 | Growth inhibition assay | Inhibition of human HSC-3 cell growth in a cell viability assay, IC50 = 0.005619 μM. | SANGER | |||
| SW872 | Growth inhibition assay | Inhibition of human SW872 cell growth in a cell viability assay, IC50 = 0.00565 μM. | SANGER | |||
| Calu-6 | Growth inhibition assay | Inhibition of human Calu-6 cell growth in a cell viability assay, IC50 = 0.005701 μM. | SANGER | |||
| SK-MES-1 | Growth inhibition assay | Inhibition of human SK-MES-1 cell growth in a cell viability assay, IC50 = 0.005725 μM. | SANGER | |||
| KARPAS-45 | Growth inhibition assay | Inhibition of human KARPAS-45 cell growth in a cell viability assay, IC50 = 0.005735 μM. | SANGER | |||
| GI-ME-N | Growth inhibition assay | Inhibition of human GI-ME-N cell growth in a cell viability assay, IC50 = 0.005755 μM. | SANGER | |||
| EFO-27 | Growth inhibition assay | Inhibition of human EFO-27 cell growth in a cell viability assay, IC50 = 0.00579 μM. | SANGER | |||
| SW48 | Growth inhibition assay | Inhibition of human SW48 cell growth in a cell viability assay, IC50 = 0.0058 μM. | SANGER | |||
| MIA-PaCa-2 | Growth inhibition assay | Inhibition of human MIA-PaCa-2 cell growth in a cell viability assay, IC50 = 0.005828 μM. | SANGER | |||
| SR | Growth inhibition assay | Inhibition of human SR cell growth in a cell viability assay, IC50 = 0.005842 μM. | SANGER | |||
| NCI-H358 | Growth inhibition assay | Inhibition of human NCI-H358 cell growth in a cell viability assay, IC50 = 0.005888 μM. | SANGER | |||
| NALM-6 | Growth inhibition assay | Inhibition of human NALM-6 cell growth in a cell viability assay, IC50 = 0.00594 μM. | SANGER | |||
| PANC-03-27 | Growth inhibition assay | Inhibition of human PANC-03-27 cell growth in a cell viability assay, IC50 = 0.005948 μM. | SANGER | |||
| SW954 | Growth inhibition assay | Inhibition of human SW954 cell growth in a cell viability assay, IC50 = 0.005983 μM. | SANGER | |||
| MFE-296 | Growth inhibition assay | Inhibition of human MFE-296 cell growth in a cell viability assay, IC50 = 0.005989 μM. | SANGER | |||
| NUGC-3 | Growth inhibition assay | Inhibition of human NUGC-3 cell growth in a cell viability assay, IC50 = 0.005992 μM. | SANGER | |||
| EFM-19 | Growth inhibition assay | Inhibition of human EFM-19 cell growth in a cell viability assay, IC50 = 0.006035 μM. | SANGER | |||
| A388 | Growth inhibition assay | Inhibition of human A388 cell growth in a cell viability assay, IC50 = 0.006067 μM. | SANGER | |||
| OVCAR-3 | Growth inhibition assay | Inhibition of human OVCAR-3 cell growth in a cell viability assay, IC50 = 0.00621 μM. | SANGER | |||
| KYSE-410 | Growth inhibition assay | Inhibition of human KYSE-410 cell growth in a cell viability assay, IC50 = 0.006213 μM. | SANGER | |||
| LU-99A | Growth inhibition assay | Inhibition of human LU-99A cell growth in a cell viability assay, IC50 = 0.006239 μM. | SANGER | |||
| AN3-CA | Growth inhibition assay | Inhibition of human AN3-CA cell growth in a cell viability assay, IC50 = 0.006269 μM. | SANGER | |||
| OPM-2 | Growth inhibition assay | Inhibition of human OPM-2 cell growth in a cell viability assay, IC50 = 0.006273 μM. | SANGER | |||
| NCI-H69 | Growth inhibition assay | Inhibition of human NCI-H69 cell growth in a cell viability assay, IC50 = 0.006345 μM. | SANGER | |||
| A2780 | Growth inhibition assay | Inhibition of human A2780 cell growth in a cell viability assay, IC50 = 0.006382 μM. | SANGER | |||
| AGS | Growth inhibition assay | Inhibition of human AGS cell growth in a cell viability assay, IC50 = 0.006462 μM. | SANGER | |||
| OVCAR-8 | Growth inhibition assay | Inhibition of human OVCAR-8 cell growth in a cell viability assay, IC50 = 0.006601 μM. | SANGER | |||
| 639-V | Growth inhibition assay | Inhibition of human 639-V cell growth in a cell viability assay, IC50 = 0.006609 μM. | SANGER | |||
| EW-13 | Growth inhibition assay | Inhibition of human EW-13 cell growth in a cell viability assay, IC50 = 0.006617 μM. | SANGER | |||
| Daoy | Growth inhibition assay | Inhibition of human Daoy cell growth in a cell viability assay, IC50 = 0.006646 μM. | SANGER | |||
| HO-1-N-1 | Growth inhibition assay | Inhibition of human HO-1-N-1 cell growth in a cell viability assay, IC50 = 0.006652 μM. | SANGER | |||
| MOLT-4 | Growth inhibition assay | Inhibition of human MOLT-4 cell growth in a cell viability assay, IC50 = 0.006659 μM. | SANGER | |||
| ChaGo-K-1 | Growth inhibition assay | Inhibition of human ChaGo-K-1 cell growth in a cell viability assay, IC50 = 0.006686 μM. | SANGER | |||
| EB2 | Growth inhibition assay | Inhibition of human EB2 cell growth in a cell viability assay, IC50 = 0.0067 μM. | SANGER | |||
| J-RT3-T3-5 | Growth inhibition assay | Inhibition of human J-RT3-T3-5 cell growth in a cell viability assay, IC50 = 0.006831 μM. | SANGER | |||
| RPMI-6666 | Growth inhibition assay | Inhibition of human RPMI-6666 cell growth in a cell viability assay, IC50 = 0.006862 μM. | SANGER | |||
| MHH-PREB-1 | Growth inhibition assay | Inhibition of human MHH-PREB-1 cell growth in a cell viability assay, IC50 = 0.006884 μM. | SANGER | |||
| U251 | Growth inhibition assay | Inhibition of human U251 cell growth in a cell viability assay, IC50 = 0.006887 μM. | SANGER | |||
| LB647-SCLC | Growth inhibition assay | Inhibition of human LB647-SCLC cell growth in a cell viability assay, IC50 = 0.006889 μM. | SANGER | |||
| ETK-1 | Growth inhibition assay | Inhibition of human ETK-1 cell growth in a cell viability assay, IC50 = 0.006921 μM. | SANGER | |||
| 23132-87 | Growth inhibition assay | Inhibition of human 23132-87 cell growth in a cell viability assay, IC50 = 0.006945 μM. | SANGER | |||
| NCI-H460 | Growth inhibition assay | Inhibition of human NCI-H460 cell growth in a cell viability assay, IC50 = 0.006983 μM. | SANGER | |||
| RPMI-8866 | Growth inhibition assay | Inhibition of human RPMI-8866 cell growth in a cell viability assay, IC50 = 0.006997 μM. | SANGER | |||
| EW-18 | Growth inhibition assay | Inhibition of human EW-18 cell growth in a cell viability assay, IC50 = 0.007046 μM. | SANGER | |||
| KGN | Growth inhibition assay | Inhibition of human KGN cell growth in a cell viability assay, IC50 = 0.007051 μM. | SANGER | |||
| HuO9 | Growth inhibition assay | Inhibition of human HuO9 cell growth in a cell viability assay, IC50 = 0.007092 μM. | SANGER | |||
| NCI-H1437 | Growth inhibition assay | Inhibition of human NCI-H1437 cell growth in a cell viability assay, IC50 = 0.007111 μM. | SANGER | |||
| RL95-2 | Growth inhibition assay | Inhibition of human RL95-2 cell growth in a cell viability assay, IC50 = 0.007132 μM. | SANGER | |||
| KYSE-180 | Growth inhibition assay | Inhibition of human KYSE-180 cell growth in a cell viability assay, IC50 = 0.007134 μM. | SANGER | |||
| MDA-MB-468 | Growth inhibition assay | Inhibition of human MDA-MB-468 cell growth in a cell viability assay, IC50 = 0.007137 μM. | SANGER | |||
| SK-MEL-2 | Growth inhibition assay | Inhibition of human SK-MEL-2 cell growth in a cell viability assay, IC50 = 0.007183 μM. | SANGER | |||
| LC4-1 | Growth inhibition assay | Inhibition of human LC4-1 cell growth in a cell viability assay, IC50 = 0.007188 μM. | SANGER | |||
| TUR | Growth inhibition assay | Inhibition of human TUR cell growth in a cell viability assay, IC50 = 0.007198 μM. | SANGER | |||
| REH | Growth inhibition assay | Inhibition of human REH cell growth in a cell viability assay, IC50 = 0.007207 μM. | SANGER | |||
| A673 | Growth inhibition assay | Inhibition of human A673 cell growth in a cell viability assay, IC50 = 0.007268 μM. | SANGER | |||
| NCI-H64 | Growth inhibition assay | Inhibition of human NCI-H64 cell growth in a cell viability assay, IC50 = 0.007273 μM. | SANGER | |||
| C-33-A | Growth inhibition assay | Inhibition of human C-33-A cell growth in a cell viability assay, IC50 = 0.007314 μM. | SANGER | |||
| Detroit562 | Growth inhibition assay | Inhibition of human Detroit562 cell growth in a cell viability assay, IC50 = 0.007549 μM. | SANGER | |||
| 5637 | Growth inhibition assay | Inhibition of human 5637 cell growth in a cell viability assay, IC50 = 0.007552 μM. | SANGER | |||
| SNU-C2B | Growth inhibition assay | Inhibition of human SNU-C2B cell growth in a cell viability assay, IC50 = 0.007749 μM. | SANGER | |||
| GP5d | Growth inhibition assay | Inhibition of human GP5d cell growth in a cell viability assay, IC50 = 0.007841 μM. | SANGER | |||
| H4 | Growth inhibition assay | Inhibition of human H4 cell growth in a cell viability assay, IC50 = 0.007851 μM. | SANGER | |||
| G-401 | Growth inhibition assay | Inhibition of human G-401 cell growth in a cell viability assay, IC50 = 0.007874 μM. | SANGER | |||
| LoVo | Growth inhibition assay | Inhibition of human LoVo cell growth in a cell viability assay, IC50 = 0.007902 μM. | SANGER | |||
| RS4-11 | Growth inhibition assay | Inhibition of human RS4-11 cell growth in a cell viability assay, IC50 = 0.00799 μM. | SANGER | |||
| KNS-42 | Growth inhibition assay | Inhibition of human KNS-42 cell growth in a cell viability assay, IC50 = 0.008028 μM. | SANGER | |||
| NY | Growth inhibition assay | Inhibition of human NY cell growth in a cell viability assay, IC50 = 0.008123 μM. | SANGER | |||
| CAL-62 | Growth inhibition assay | Inhibition of human CAL-62 cell growth in a cell viability assay, IC50 = 0.008183 μM. | SANGER | |||
| AU565 | Growth inhibition assay | Inhibition of human AU565 cell growth in a cell viability assay, IC50 = 0.008185 μM. | SANGER | |||
| NCI-H2087 | Growth inhibition assay | Inhibition of human NCI-H2087 cell growth in a cell viability assay, IC50 = 0.008218 μM. | SANGER | |||
| GR-ST | Growth inhibition assay | Inhibition of human GR-ST cell growth in a cell viability assay, IC50 = 0.008272 μM. | SANGER | |||
| CCF-STTG1 | Growth inhibition assay | Inhibition of human CCF-STTG1 cell growth in a cell viability assay, IC50 = 0.008373 μM. | SANGER | |||
| SK-OV-3 | Growth inhibition assay | Inhibition of human SK-OV-3 cell growth in a cell viability assay, IC50 = 0.008386 μM. | SANGER | |||
| NCI-H2009 | Growth inhibition assay | Inhibition of human NCI-H2009 cell growth in a cell viability assay, IC50 = 0.008458 μM. | SANGER | |||
| SK-CO-1 | Growth inhibition assay | Inhibition of human SK-CO-1 cell growth in a cell viability assay, IC50 = 0.008497 μM. | SANGER | |||
| SW620 | Growth inhibition assay | Inhibition of human SW620 cell growth in a cell viability assay, IC50 = 0.008523 μM. | SANGER | |||
| LB771-HNC | Growth inhibition assay | Inhibition of human LB771-HNC cell growth in a cell viability assay, IC50 = 0.008591 μM. | SANGER | |||
| CAL-51 | Growth inhibition assay | Inhibition of human CAL-51 cell growth in a cell viability assay, IC50 = 0.008613 μM. | SANGER | |||
| A101D | Growth inhibition assay | Inhibition of human A101D cell growth in a cell viability assay, IC50 = 0.008674 μM. | SANGER | |||
| NCI-H526 | Growth inhibition assay | Inhibition of human NCI-H526 cell growth in a cell viability assay, IC50 = 0.008675 μM. | SANGER | |||
| SCC-15 | Growth inhibition assay | Inhibition of human SCC-15 cell growth in a cell viability assay, IC50 = 0.008695 μM. | SANGER | |||
| 22RV1 | Growth inhibition assay | Inhibition of human 22RV1 cell growth in a cell viability assay, IC50 = 0.008787 μM. | SANGER | |||
| RPMI-7951 | Growth inhibition assay | Inhibition of human RPMI-7951 cell growth in a cell viability assay, IC50 = 0.008815 μM. | SANGER | |||
| HH | Growth inhibition assay | Inhibition of human HH cell growth in a cell viability assay, IC50 = 0.008879 μM. | SANGER | |||
| SK-NEP-1 | Growth inhibition assay | Inhibition of human SK-NEP-1 cell growth in a cell viability assay, IC50 = 0.00899 μM. | SANGER | |||
| EoL-1-cell | Growth inhibition assay | Inhibition of human EoL-1-cell cell growth in a cell viability assay, IC50 = 0.009086 μM. | SANGER | |||
| KM-H2 | Growth inhibition assay | Inhibition of human KM-H2 cell growth in a cell viability assay, IC50 = 0.009108 μM. | SANGER | |||
| ESS-1 | Growth inhibition assay | Inhibition of human ESS-1 cell growth in a cell viability assay, IC50 = 0.009136 μM. | SANGER | |||
| TE-11 | Growth inhibition assay | Inhibition of human TE-11 cell growth in a cell viability assay, IC50 = 0.009155 μM. | SANGER | |||
| MC-CAR | Growth inhibition assay | Inhibition of human MC-CAR cell growth in a cell viability assay, IC50 = 0.009274 μM. | SANGER | |||
| KU812 | Growth inhibition assay | Inhibition of human KU812 cell growth in a cell viability assay, IC50 = 0.009301 μM. | SANGER | |||
| BC-1 | Growth inhibition assay | Inhibition of human BC-1 cell growth in a cell viability assay, IC50 = 0.009391 μM. | SANGER | |||
| SU-DHL-1 | Growth inhibition assay | Inhibition of human SU-DHL-1 cell growth in a cell viability assay, IC50 = 0.009451 μM. | SANGER | |||
| LOUCY | Growth inhibition assay | Inhibition of human LOUCY cell growth in a cell viability assay, IC50 = 0.009689 μM. | SANGER | |||
| KYSE-450 | Growth inhibition assay | Inhibition of human KYSE-450 cell growth in a cell viability assay, IC50 = 0.009741 μM. | SANGER | |||
| MDA-MB-361 | Growth inhibition assay | Inhibition of human MDA-MB-361 cell growth in a cell viability assay, IC50 = 0.009759 μM. | SANGER | |||
| COR-L23 | Growth inhibition assay | Inhibition of human COR-L23 cell growth in a cell viability assay, IC50 = 0.009886 μM. | SANGER | |||
| BHY | Growth inhibition assay | Inhibition of human BHY cell growth in a cell viability assay, IC50 = 0.009946 μM. | SANGER | |||
| RPMI-2650 | Growth inhibition assay | Inhibition of human RPMI-2650 cell growth in a cell viability assay, IC50 = 0.009996 μM. | SANGER | |||
| HCC1954 | Growth inhibition assay | Inhibition of human HCC1954 cell growth in a cell viability assay, IC50 = 0.01009 μM. | SANGER | |||
| P30-OHK | Growth inhibition assay | Inhibition of human P30-OHK cell growth in a cell viability assay, IC50 = 0.0101 μM. | SANGER | |||
| RERF-LC-MS | Growth inhibition assay | Inhibition of human RERF-LC-MS cell growth in a cell viability assay, IC50 = 0.01018 μM. | SANGER | |||
| HLE | Growth inhibition assay | Inhibition of human HLE cell growth in a cell viability assay, IC50 = 0.01021 μM. | SANGER | |||
| SNU-387 | Growth inhibition assay | Inhibition of human SNU-387 cell growth in a cell viability assay, IC50 = 0.01023 μM. | SANGER | |||
| MZ7-mel | Growth inhibition assay | Inhibition of human MZ7-mel cell growth in a cell viability assay, IC50 = 0.01024 μM. | SANGER | |||
| OCI-AML2 | Growth inhibition assay | Inhibition of human OCI-AML2 cell growth in a cell viability assay, IC50 = 0.01025 μM. | SANGER | |||
| Ca9-22 | Growth inhibition assay | Inhibition of human Ca9-22 cell growth in a cell viability assay, IC50 = 0.01027 μM. | SANGER | |||
| MV-4-11 | Growth inhibition assay | Inhibition of human MV-4-11 cell growth in a cell viability assay, IC50 = 0.01039 μM. | SANGER | |||
| HCC1599 | Growth inhibition assay | Inhibition of human HCC1599 cell growth in a cell viability assay, IC50 = 0.01057 μM. | SANGER | |||
| JiyoyeP-2003 | Growth inhibition assay | Inhibition of human JiyoyeP-2003 cell growth in a cell viability assay, IC50 = 0.01073 μM. | SANGER | |||
| BFTC-909 | Growth inhibition assay | Inhibition of human BFTC-909 cell growth in a cell viability assay, IC50 = 0.01078 μM. | SANGER | |||
| RH-1 | Growth inhibition assay | Inhibition of human RH-1 cell growth in a cell viability assay, IC50 = 0.01102 μM. | SANGER | |||
| IMR-5 | Growth inhibition assay | Inhibition of human IMR-5 cell growth in a cell viability assay, IC50 = 0.01125 μM. | SANGER | |||
| NCI-H2170 | Growth inhibition assay | Inhibition of human NCI-H2170 cell growth in a cell viability assay, IC50 = 0.0116 μM. | SANGER | |||
| TE-5 | Growth inhibition assay | Inhibition of human TE-5 cell growth in a cell viability assay, IC50 = 0.01169 μM. | SANGER | |||
| PF-382 | Growth inhibition assay | Inhibition of human PF-382 cell growth in a cell viability assay, IC50 = 0.01172 μM. | SANGER | |||
| MKN1 | Growth inhibition assay | Inhibition of human MKN1 cell growth in a cell viability assay, IC50 = 0.01177 μM. | SANGER | |||
| SF295 | Growth inhibition assay | Inhibition of human SF295 cell growth in a cell viability assay, IC50 = 0.01203 μM. | SANGER | |||
| U-698-M | Growth inhibition assay | Inhibition of human U-698-M cell growth in a cell viability assay, IC50 = 0.01217 μM. | SANGER | |||
| RPMI-8402 | Growth inhibition assay | Inhibition of human RPMI-8402 cell growth in a cell viability assay, IC50 = 0.0123 μM. | SANGER | |||
| BT-20 | Growth inhibition assay | Inhibition of human BT-20 cell growth in a cell viability assay, IC50 = 0.01236 μM. | SANGER | |||
| BE-13 | Growth inhibition assay | Inhibition of human BE-13 cell growth in a cell viability assay, IC50 = 0.01237 μM. | SANGER | |||
| L-363 | Growth inhibition assay | Inhibition of human L-363 cell growth in a cell viability assay, IC50 = 0.01238 μM. | SANGER | |||
| SK-MEL-30 | Growth inhibition assay | Inhibition of human SK-MEL-30 cell growth in a cell viability assay, IC50 = 0.01241 μM. | SANGER | |||
| SNB75 | Growth inhibition assay | Inhibition of human SNB75 cell growth in a cell viability assay, IC50 = 0.01245 μM. | SANGER | |||
| SK-PN-DW | Growth inhibition assay | Inhibition of human SK-PN-DW cell growth in a cell viability assay, IC50 = 0.01247 μM. | SANGER | |||
| SCC-25 | Growth inhibition assay | Inhibition of human SCC-25 cell growth in a cell viability assay, IC50 = 0.01248 μM. | SANGER | |||
| LK-2 | Growth inhibition assay | Inhibition of human LK-2 cell growth in a cell viability assay, IC50 = 0.01251 μM. | SANGER | |||
| D-247MG | Growth inhibition assay | Inhibition of human D-247MG cell growth in a cell viability assay, IC50 = 0.0126 μM. | SANGER | |||
| SJSA-1 | Growth inhibition assay | Inhibition of human SJSA-1 cell growth in a cell viability assay, IC50 = 0.01261 μM. | SANGER | |||
| MOLT-16 | Growth inhibition assay | Inhibition of human MOLT-16 cell growth in a cell viability assay, IC50 = 0.01278 μM. | SANGER | |||
| NCI-H1563 | Growth inhibition assay | Inhibition of human NCI-H1563 cell growth in a cell viability assay, IC50 = 0.01281 μM. | SANGER | |||
| NCI-H2228 | Growth inhibition assay | Inhibition of human NCI-H2228 cell growth in a cell viability assay, IC50 = 0.01292 μM. | SANGER | |||
| HuO-3N1 | Growth inhibition assay | Inhibition of human HuO-3N1 cell growth in a cell viability assay, IC50 = 0.01298 μM. | SANGER | |||
| GAMG | Growth inhibition assay | Inhibition of human GAMG cell growth in a cell viability assay, IC50 = 0.01303 μM. | SANGER | |||
| TE-1 | Growth inhibition assay | Inhibition of human TE-1 cell growth in a cell viability assay, IC50 = 0.01306 μM. | SANGER | |||
| OE19 | Growth inhibition assay | Inhibition of human OE19 cell growth in a cell viability assay, IC50 = 0.01324 μM. | SANGER | |||
| NCI-H1436 | Growth inhibition assay | Inhibition of human NCI-H1436 cell growth in a cell viability assay, IC50 = 0.01358 μM. | SANGER | |||
| NCI-H1299 | Growth inhibition assay | Inhibition of human NCI-H1299 cell growth in a cell viability assay, IC50 = 0.01363 μM. | SANGER | |||
| COLO-684 | Growth inhibition assay | Inhibition of human COLO-684 cell growth in a cell viability assay, IC50 = 0.01366 μM. | SANGER | |||
| CMK | Growth inhibition assay | Inhibition of human CMK cell growth in a cell viability assay, IC50 = 0.01372 μM. | SANGER | |||
| HCC1187 | Growth inhibition assay | Inhibition of human HCC1187 cell growth in a cell viability assay, IC50 = 0.01386 μM. | SANGER | |||
| NEC8 | Growth inhibition assay | Inhibition of human NEC8 cell growth in a cell viability assay, IC50 = 0.01391 μM. | SANGER | |||
| A172 | Growth inhibition assay | Inhibition of human A172 cell growth in a cell viability assay, IC50 = 0.01392 μM. | SANGER | |||
| NCI-H187 | Growth inhibition assay | Inhibition of human NCI-H187 cell growth in a cell viability assay, IC50 = 0.01402 μM. | SANGER | |||
| M059J | Growth inhibition assay | Inhibition of human M059J cell growth in a cell viability assay, IC50 = 0.01406 μM. | SANGER | |||
| JEG-3 | Growth inhibition assay | Inhibition of human JEG-3 cell growth in a cell viability assay, IC50 = 0.01407 μM. | SANGER | |||
| NCI-H1734 | Growth inhibition assay | Inhibition of human NCI-H1734 cell growth in a cell viability assay, IC50 = 0.0141 μM. | SANGER | |||
| SF539 | Growth inhibition assay | Inhibition of human SF539 cell growth in a cell viability assay, IC50 = 0.0141 μM. | SANGER | |||
| CAPAN-1 | Growth inhibition assay | Inhibition of human CAPAN-1 cell growth in a cell viability assay, IC50 = 0.01418 μM. | SANGER | |||
| NCI-H446 | Growth inhibition assay | Inhibition of human NCI-H446 cell growth in a cell viability assay, IC50 = 0.01481 μM. | SANGER | |||
| SW780 | Growth inhibition assay | Inhibition of human SW780 cell growth in a cell viability assay, IC50 = 0.01487 μM. | SANGER | |||
| NMC-G1 | Growth inhibition assay | Inhibition of human NMC-G1 cell growth in a cell viability assay, IC50 = 0.01495 μM. | SANGER | |||
| Mewo | Growth inhibition assay | Inhibition of human Mewo cell growth in a cell viability assay, IC50 = 0.01518 μM. | SANGER | |||
| OAW-28 | Growth inhibition assay | Inhibition of human OAW-28 cell growth in a cell viability assay, IC50 = 0.01522 μM. | SANGER | |||
| CAL-148 | Growth inhibition assay | Inhibition of human CAL-148 cell growth in a cell viability assay, IC50 = 0.0154 μM. | SANGER | |||
| NCI-H510A | Growth inhibition assay | Inhibition of human NCI-H510A cell growth in a cell viability assay, IC50 = 0.01545 μM. | SANGER | |||
| SH-4 | Growth inhibition assay | Inhibition of human SH-4 cell growth in a cell viability assay, IC50 = 0.01552 μM. | SANGER | |||
| MC116 | Growth inhibition assay | Inhibition of human MC116 cell growth in a cell viability assay, IC50 = 0.01554 μM. | SANGER | |||
| DJM-1 | Growth inhibition assay | Inhibition of human DJM-1 cell growth in a cell viability assay, IC50 = 0.01587 μM. | SANGER | |||
| HCC1395 | Growth inhibition assay | Inhibition of human HCC1395 cell growth in a cell viability assay, IC50 = 0.01587 μM. | SANGER | |||
| HDLM-2 | Growth inhibition assay | Inhibition of human HDLM-2 cell growth in a cell viability assay, IC50 = 0.0159 μM. | SANGER | |||
| NCI-H838 | Growth inhibition assay | Inhibition of human NCI-H838 cell growth in a cell viability assay, IC50 = 0.01604 μM. | SANGER | |||
| NCI-H650 | Growth inhibition assay | Inhibition of human NCI-H650 cell growth in a cell viability assay, IC50 = 0.01608 μM. | SANGER | |||
| SF268 | Growth inhibition assay | Inhibition of human SF268 cell growth in a cell viability assay, IC50 = 0.01631 μM. | SANGER | |||
| HPAF-II | Growth inhibition assay | Inhibition of human HPAF-II cell growth in a cell viability assay, IC50 = 0.01662 μM. | SANGER | |||
| KYSE-70 | Growth inhibition assay | Inhibition of human KYSE-70 cell growth in a cell viability assay, IC50 = 0.01669 μM. | SANGER | |||
| EW-7 | Growth inhibition assay | Inhibition of human EW-7 cell growth in a cell viability assay, IC50 = 0.0167 μM. | SANGER | |||
| 769-P | Growth inhibition assay | Inhibition of human 769-P cell growth in a cell viability assay, IC50 = 0.01692 μM. | SANGER | |||
| COLO-800 | Growth inhibition assay | Inhibition of human COLO-800 cell growth in a cell viability assay, IC50 = 0.01695 μM. | SANGER | |||
| RPMI-8226 | Growth inhibition assay | Inhibition of human RPMI-8226 cell growth in a cell viability assay, IC50 = 0.01703 μM. | SANGER | |||
| IST-SL2 | Growth inhibition assay | Inhibition of human IST-SL2 cell growth in a cell viability assay, IC50 = 0.01714 μM. | SANGER | |||
| SK-MEL-3 | Growth inhibition assay | Inhibition of human SK-MEL-3 cell growth in a cell viability assay, IC50 = 0.01733 μM. | SANGER | |||
| NCI-H1755 | Growth inhibition assay | Inhibition of human NCI-H1755 cell growth in a cell viability assay, IC50 = 0.01736 μM. | SANGER | |||
| U-266 | Growth inhibition assay | Inhibition of human U-266 cell growth in a cell viability assay, IC50 = 0.01747 μM. | SANGER | |||
| C8166 | Growth inhibition assay | Inhibition of human C8166 cell growth in a cell viability assay, IC50 = 0.01756 μM. | SANGER | |||
| MHH-NB-11 | Growth inhibition assay | Inhibition of human MHH-NB-11 cell growth in a cell viability assay, IC50 = 0.01765 μM. | SANGER | |||
| KASUMI-1 | Growth inhibition assay | Inhibition of human KASUMI-1 cell growth in a cell viability assay, IC50 = 0.01769 μM. | SANGER | |||
| MZ1-PC | Growth inhibition assay | Inhibition of human MZ1-PC cell growth in a cell viability assay, IC50 = 0.0177 μM. | SANGER | |||
| ARH-77 | Growth inhibition assay | Inhibition of human ARH-77 cell growth in a cell viability assay, IC50 = 0.01776 μM. | SANGER | |||
| KYSE-520 | Growth inhibition assay | Inhibition of human KYSE-520 cell growth in a cell viability assay, IC50 = 0.01783 μM. | SANGER | |||
| LNCaP-Clone-FGC | Growth inhibition assay | Inhibition of human LNCaP-Clone-FGC cell growth in a cell viability assay, IC50 = 0.01793 μM. | SANGER | |||
| Calu-3 | Growth inhibition assay | Inhibition of human Calu-3 cell growth in a cell viability assay, IC50 = 0.018 μM. | SANGER | |||
| HCC2157 | Growth inhibition assay | Inhibition of human HCC2157 cell growth in a cell viability assay, IC50 = 0.0188 μM. | SANGER | |||
| VMRC-RCZ | Growth inhibition assay | Inhibition of human VMRC-RCZ cell growth in a cell viability assay, IC50 = 0.01882 μM. | SANGER | |||
| T98G | Growth inhibition assay | Inhibition of human T98G cell growth in a cell viability assay, IC50 = 0.01891 μM. | SANGER | |||
| NOMO-1 | Growth inhibition assay | Inhibition of human NOMO-1 cell growth in a cell viability assay, IC50 = 0.01902 μM. | SANGER | |||
| VM-CUB-1 | Growth inhibition assay | Inhibition of human VM-CUB-1 cell growth in a cell viability assay, IC50 = 0.01907 μM. | SANGER | |||
| Ca-Ski | Growth inhibition assay | Inhibition of human Ca-Ski cell growth in a cell viability assay, IC50 = 0.01912 μM. | SANGER | |||
| NCI-H1793 | Growth inhibition assay | Inhibition of human NCI-H1793 cell growth in a cell viability assay, IC50 = 0.01924 μM. | SANGER | |||
| DB | Growth inhibition assay | Inhibition of human DB cell growth in a cell viability assay, IC50 = 0.01941 μM. | SANGER | |||
| CTB-1 | Growth inhibition assay | Inhibition of human CTB-1 cell growth in a cell viability assay, IC50 = 0.0198 μM. | SANGER | |||
| SW948 | Growth inhibition assay | Inhibition of human SW948 cell growth in a cell viability assay, IC50 = 0.01985 μM. | SANGER | |||
| TGBC24TKB | Growth inhibition assay | Inhibition of human TGBC24TKB cell growth in a cell viability assay, IC50 = 0.02014 μM. | SANGER | |||
| NCI-H661 | Growth inhibition assay | Inhibition of human NCI-H661 cell growth in a cell viability assay, IC50 = 0.02035 μM. | SANGER | |||
| OE33 | Growth inhibition assay | Inhibition of human OE33 cell growth in a cell viability assay, IC50 = 0.02043 μM. | SANGER | |||
| MMAC-SF | Growth inhibition assay | Inhibition of human MMAC-SF cell growth in a cell viability assay, IC50 = 0.02046 μM. | SANGER | |||
| PFSK-1 | Growth inhibition assay | Inhibition of human PFSK-1 cell growth in a cell viability assay, IC50 = 0.02054 μM. | SANGER | |||
| MONO-MAC-6 | Growth inhibition assay | Inhibition of human MONO-MAC-6 cell growth in a cell viability assay, IC50 = 0.02061 μM. | SANGER | |||
| CAL-12T | Growth inhibition assay | Inhibition of human CAL-12T cell growth in a cell viability assay, IC50 = 0.02076 μM. | SANGER | |||
| MEG-01 | Growth inhibition assay | Inhibition of human MEG-01 cell growth in a cell viability assay, IC50 = 0.0208 μM. | SANGER | |||
| HCE-4 | Growth inhibition assay | Inhibition of human HCE-4 cell growth in a cell viability assay, IC50 = 0.02085 μM. | SANGER | |||
| 647-V | Growth inhibition assay | Inhibition of human 647-V cell growth in a cell viability assay, IC50 = 0.02089 μM. | SANGER | |||
| ME-180 | Growth inhibition assay | Inhibition of human ME-180 cell growth in a cell viability assay, IC50 = 0.02104 μM. | SANGER | |||
| M14 | Growth inhibition assay | Inhibition of human M14 cell growth in a cell viability assay, IC50 = 0.02112 μM. | SANGER | |||
| K052 | Growth inhibition assay | Inhibition of human K052 cell growth in a cell viability assay, IC50 = 0.02119 μM. | SANGER | |||
| UM-UC-3 | Growth inhibition assay | Inhibition of human UM-UC-3 cell growth in a cell viability assay, IC50 = 0.02122 μM. | SANGER | |||
| A3-KAW | Growth inhibition assay | Inhibition of human A3-KAW cell growth in a cell viability assay, IC50 = 0.02157 μM. | SANGER | |||
| HT | Growth inhibition assay | Inhibition of human HT cell growth in a cell viability assay, IC50 = 0.02158 μM. | SANGER | |||
| EFO-21 | Growth inhibition assay | Inhibition of human EFO-21 cell growth in a cell viability assay, IC50 = 0.02171 μM. | SANGER | |||
| KP-N-YS | Growth inhibition assay | Inhibition of human KP-N-YS cell growth in a cell viability assay, IC50 = 0.02183 μM. | SANGER | |||
| CA46 | Growth inhibition assay | Inhibition of human CA46 cell growth in a cell viability assay, IC50 = 0.02217 μM. | SANGER | |||
| HEC-1 | Growth inhibition assay | Inhibition of human HEC-1 cell growth in a cell viability assay, IC50 = 0.02272 μM. | SANGER | |||
| C-4-II | Growth inhibition assay | Inhibition of human C-4-II cell growth in a cell viability assay, IC50 = 0.02301 μM. | SANGER | |||
| KARPAS-422 | Growth inhibition assay | Inhibition of human KARPAS-422 cell growth in a cell viability assay, IC50 = 0.02329 μM. | SANGER | |||
| KYSE-140 | Growth inhibition assay | Inhibition of human KYSE-140 cell growth in a cell viability assay, IC50 = 0.02384 μM. | SANGER | |||
| HCC1806 | Growth inhibition assay | Inhibition of human HCC1806 cell growth in a cell viability assay, IC50 = 0.02389 μM. | SANGER | |||
| NCI-H1417 | Growth inhibition assay | Inhibition of human NCI-H1417 cell growth in a cell viability assay, IC50 = 0.02406 μM. | SANGER | |||
| MDA-MB-415 | Growth inhibition assay | Inhibition of human MDA-MB-415 cell growth in a cell viability assay, IC50 = 0.0241 μM. | SANGER | |||
| SW684 | Growth inhibition assay | Inhibition of human SW684 cell growth in a cell viability assay, IC50 = 0.02416 μM. | SANGER | |||
| LU-65 | Growth inhibition assay | Inhibition of human LU-65 cell growth in a cell viability assay, IC50 = 0.02449 μM. | SANGER | |||
| CHP-212 | Growth inhibition assay | Inhibition of human CHP-212 cell growth in a cell viability assay, IC50 = 0.02459 μM. | SANGER | |||
| GDM-1 | Growth inhibition assay | Inhibition of human GDM-1 cell growth in a cell viability assay, IC50 = 0.02461 μM. | SANGER | |||
| NCI-H226 | Growth inhibition assay | Inhibition of human NCI-H226 cell growth in a cell viability assay, IC50 = 0.02495 μM. | SANGER | |||
| P12-ICHIKAWA | Growth inhibition assay | Inhibition of human P12-ICHIKAWA cell growth in a cell viability assay, IC50 = 0.02515 μM. | SANGER | |||
| NB1 | Growth inhibition assay | Inhibition of human NB1 cell growth in a cell viability assay, IC50 = 0.02608 μM. | SANGER | |||
| NCI-H2347 | Growth inhibition assay | Inhibition of human NCI-H2347 cell growth in a cell viability assay, IC50 = 0.02611 μM. | SANGER | |||
| A498 | Growth inhibition assay | Inhibition of human A498 cell growth in a cell viability assay, IC50 = 0.02621 μM. | SANGER | |||
| HTC-C3 | Growth inhibition assay | Inhibition of human HTC-C3 cell growth in a cell viability assay, IC50 = 0.02649 μM. | SANGER | |||
| NH-12 | Growth inhibition assay | Inhibition of human NH-12 cell growth in a cell viability assay, IC50 = 0.02702 μM. | SANGER | |||
| TCCSUP | Growth inhibition assay | Inhibition of human TCCSUP cell growth in a cell viability assay, IC50 = 0.02706 μM. | SANGER | |||
| U-87-MG | Growth inhibition assay | Inhibition of human U-87-MG cell growth in a cell viability assay, IC50 = 0.02709 μM. | SANGER | |||
| SW962 | Growth inhibition assay | Inhibition of human SW962 cell growth in a cell viability assay, IC50 = 0.02783 μM. | SANGER | |||
| ES3 | Growth inhibition assay | Inhibition of human ES3 cell growth in a cell viability assay, IC50 = 0.02815 μM. | SANGER | |||
| BT-474 | Growth inhibition assay | Inhibition of human BT-474 cell growth in a cell viability assay, IC50 = 0.02848 μM. | SANGER | |||
| NCI-H1623 | Growth inhibition assay | Inhibition of human NCI-H1623 cell growth in a cell viability assay, IC50 = 0.02849 μM. | SANGER | |||
| COLO-668 | Growth inhibition assay | Inhibition of human COLO-668 cell growth in a cell viability assay, IC50 = 0.0285 μM. | SANGER | |||
| LB831-BLC | Growth inhibition assay | Inhibition of human LB831-BLC cell growth in a cell viability assay, IC50 = 0.02915 μM. | SANGER | |||
| HD-MY-Z | Growth inhibition assay | Inhibition of human HD-MY-Z cell growth in a cell viability assay, IC50 = 0.02924 μM. | SANGER | |||
| SJRH30 | Growth inhibition assay | Inhibition of human SJRH30 cell growth in a cell viability assay, IC50 = 0.02931 μM. | SANGER | |||
| NCI-H2126 | Growth inhibition assay | Inhibition of human NCI-H2126 cell growth in a cell viability assay, IC50 = 0.02932 μM. | SANGER | |||
| HuH-7 | Growth inhibition assay | Inhibition of human HuH-7 cell growth in a cell viability assay, IC50 = 0.02957 μM. | SANGER | |||
| L-540 | Growth inhibition assay | Inhibition of human L-540 cell growth in a cell viability assay, IC50 = 0.03002 μM. | SANGER | |||
| NCI-H1963 | Growth inhibition assay | Inhibition of human NCI-H1963 cell growth in a cell viability assay, IC50 = 0.03032 μM. | SANGER | |||
| BV-173 | Growth inhibition assay | Inhibition of human BV-173 cell growth in a cell viability assay, IC50 = 0.03041 μM. | SANGER | |||
| UACC-257 | Growth inhibition assay | Inhibition of human UACC-257 cell growth in a cell viability assay, IC50 = 0.03044 μM. | SANGER | |||
| Capan-2 | Growth inhibition assay | Inhibition of human Capan-2 cell growth in a cell viability assay, IC50 = 0.03045 μM. | SANGER | |||
| HCC70 | Growth inhibition assay | Inhibition of human HCC70 cell growth in a cell viability assay, IC50 = 0.03057 μM. | SANGER | |||
| COR-L105 | Growth inhibition assay | Inhibition of human COR-L105 cell growth in a cell viability assay, IC50 = 0.03094 μM. | SANGER | |||
| BC-3 | Growth inhibition assay | Inhibition of human BC-3 cell growth in a cell viability assay, IC50 = 0.03112 μM. | SANGER | |||
| HT-1376 | Growth inhibition assay | Inhibition of human HT-1376 cell growth in a cell viability assay, IC50 = 0.03146 μM. | SANGER | |||
| CAL-27 | Growth inhibition assay | Inhibition of human CAL-27 cell growth in a cell viability assay, IC50 = 0.03204 μM. | SANGER | |||
| G-402 | Growth inhibition assay | Inhibition of human G-402 cell growth in a cell viability assay, IC50 = 0.03208 μM. | SANGER | |||
| NCI-SNU-1 | Growth inhibition assay | Inhibition of human NCI-SNU-1 cell growth in a cell viability assay, IC50 = 0.03275 μM. | SANGER | |||
| NCI-H441 | Growth inhibition assay | Inhibition of human NCI-H441 cell growth in a cell viability assay, IC50 = 0.03312 μM. | SANGER | |||
| SN12C | Growth inhibition assay | Inhibition of human SN12C cell growth in a cell viability assay, IC50 = 0.03312 μM. | SANGER | |||
| UACC-62 | Growth inhibition assay | Inhibition of human UACC-62 cell growth in a cell viability assay, IC50 = 0.03345 μM. | SANGER | |||
| AM-38 | Growth inhibition assay | Inhibition of human AM-38 cell growth in a cell viability assay, IC50 = 0.03355 μM. | SANGER | |||
| NCI-H2081 | Growth inhibition assay | Inhibition of human NCI-H2081 cell growth in a cell viability assay, IC50 = 0.03362 μM. | SANGER | |||
| WSU-NHL | Growth inhibition assay | Inhibition of human WSU-NHL cell growth in a cell viability assay, IC50 = 0.03365 μM. | SANGER | |||
| SW1573 | Growth inhibition assay | Inhibition of human SW1573 cell growth in a cell viability assay, IC50 = 0.034 μM. | SANGER | |||
| OS-RC-2 | Growth inhibition assay | Inhibition of human OS-RC-2 cell growth in a cell viability assay, IC50 = 0.03434 μM. | SANGER | |||
| DV-90 | Growth inhibition assay | Inhibition of human DV-90 cell growth in a cell viability assay, IC50 = 0.03448 μM. | SANGER | |||
| COR-L88 | Growth inhibition assay | Inhibition of human COR-L88 cell growth in a cell viability assay, IC50 = 0.03482 μM. | SANGER | |||
| TE-441-T | Growth inhibition assay | Inhibition of human TE-441-T cell growth in a cell viability assay, IC50 = 0.03486 μM. | SANGER | |||
| NCI-H1304 | Growth inhibition assay | Inhibition of human NCI-H1304 cell growth in a cell viability assay, IC50 = 0.03516 μM. | SANGER | |||
| COLO-741 | Growth inhibition assay | Inhibition of human COLO-741 cell growth in a cell viability assay, IC50 = 0.03569 μM. | SANGER | |||
| NCI-H1882 | Growth inhibition assay | Inhibition of human NCI-H1882 cell growth in a cell viability assay, IC50 = 0.03654 μM. | SANGER | |||
| IPC-298 | Growth inhibition assay | Inhibition of human IPC-298 cell growth in a cell viability assay, IC50 = 0.03656 μM. | SANGER | |||
| MLMA | Growth inhibition assay | Inhibition of human MLMA cell growth in a cell viability assay, IC50 = 0.0369 μM. | SANGER | |||
| NCI-H1581 | Growth inhibition assay | Inhibition of human NCI-H1581 cell growth in a cell viability assay, IC50 = 0.03716 μM. | SANGER | |||
| HuP-T4 | Growth inhibition assay | Inhibition of human HuP-T4 cell growth in a cell viability assay, IC50 = 0.0373 μM. | SANGER | |||
| MDA-MB-231 | Growth inhibition assay | Inhibition of human MDA-MB-231 cell growth in a cell viability assay, IC50 = 0.03739 μM. | SANGER | |||
| SW626 | Growth inhibition assay | Inhibition of human SW626 cell growth in a cell viability assay, IC50 = 0.03767 μM. | SANGER | |||
| A375 | Growth inhibition assay | Inhibition of human A375 cell growth in a cell viability assay, IC50 = 0.03821 μM. | SANGER | |||
| HEL | Growth inhibition assay | Inhibition of human HEL cell growth in a cell viability assay, IC50 = 0.03828 μM. | SANGER | |||
| NCI-SNU-16 | Growth inhibition assay | Inhibition of human NCI-SNU-16 cell growth in a cell viability assay, IC50 = 0.03856 μM. | SANGER | |||
| NCI-H2141 | Growth inhibition assay | Inhibition of human NCI-H2141 cell growth in a cell viability assay, IC50 = 0.03899 μM. | SANGER | |||
| IST-SL1 | Growth inhibition assay | Inhibition of human IST-SL1 cell growth in a cell viability assay, IC50 = 0.03932 μM. | SANGER | |||
| CAS-1 | Growth inhibition assay | Inhibition of human CAS-1 cell growth in a cell viability assay, IC50 = 0.04006 μM. | SANGER | |||
| NCI-H889 | Growth inhibition assay | Inhibition of human NCI-H889 cell growth in a cell viability assay, IC50 = 0.04027 μM. | SANGER | |||
| NCI-H1650 | Growth inhibition assay | Inhibition of human NCI-H1650 cell growth in a cell viability assay, IC50 = 0.04068 μM. | SANGER | |||
| NOS-1 | Growth inhibition assay | Inhibition of human NOS-1 cell growth in a cell viability assay, IC50 = 0.04133 μM. | SANGER | |||
| K5 | Growth inhibition assay | Inhibition of human K5 cell growth in a cell viability assay, IC50 = 0.04189 μM. | SANGER | |||
| ES6 | Growth inhibition assay | Inhibition of human ES6 cell growth in a cell viability assay, IC50 = 0.0424 μM. | SANGER | |||
| OAW-42 | Growth inhibition assay | Inhibition of human OAW-42 cell growth in a cell viability assay, IC50 = 0.04245 μM. | SANGER | |||
| EW-11 | Growth inhibition assay | Inhibition of human EW-11 cell growth in a cell viability assay, IC50 = 0.04268 μM. | SANGER | |||
| MZ2-MEL | Growth inhibition assay | Inhibition of human MZ2-MEL cell growth in a cell viability assay, IC50 = 0.04295 μM. | SANGER | |||
| CP50-MEL-B | Growth inhibition assay | Inhibition of human CP50-MEL-B cell growth in a cell viability assay, IC50 = 0.04344 μM. | SANGER | |||
| CAL-120 | Growth inhibition assay | Inhibition of human CAL-120 cell growth in a cell viability assay, IC50 = 0.04357 μM. | SANGER | |||
| NCI-N87 | Growth inhibition assay | Inhibition of human NCI-N87 cell growth in a cell viability assay, IC50 = 0.04409 μM. | SANGER | |||
| NCI-H1694 | Growth inhibition assay | Inhibition of human NCI-H1694 cell growth in a cell viability assay, IC50 = 0.04439 μM. | SANGER | |||
| LC-1F | Growth inhibition assay | Inhibition of human LC-1F cell growth in a cell viability assay, IC50 = 0.04479 μM. | SANGER | |||
| EM-2 | Growth inhibition assay | Inhibition of human EM-2 cell growth in a cell viability assay, IC50 = 0.0448 μM. | SANGER | |||
| NCI-H345 | Growth inhibition assay | Inhibition of human NCI-H345 cell growth in a cell viability assay, IC50 = 0.04488 μM. | SANGER | |||
| CP66-MEL | Growth inhibition assay | Inhibition of human CP66-MEL cell growth in a cell viability assay, IC50 = 0.04508 μM. | SANGER | |||
| NCI-H630 | Growth inhibition assay | Inhibition of human NCI-H630 cell growth in a cell viability assay, IC50 = 0.04551 μM. | SANGER | |||
| NB69 | Growth inhibition assay | Inhibition of human NB69 cell growth in a cell viability assay, IC50 = 0.04553 μM. | SANGER | |||
| CAMA-1 | Growth inhibition assay | Inhibition of human CAMA-1 cell growth in a cell viability assay, IC50 = 0.04557 μM. | SANGER | |||
| no-10 | Growth inhibition assay | Inhibition of human no-10 cell growth in a cell viability assay, IC50 = 0.04561 μM. | SANGER | |||
| LAMA-84 | Growth inhibition assay | Inhibition of human LAMA-84 cell growth in a cell viability assay, IC50 = 0.04575 μM. | SANGER | |||
| ACHN | Growth inhibition assay | Inhibition of human ACHN cell growth in a cell viability assay, IC50 = 0.04577 μM. | SANGER | |||
| NCI-SNU-5 | Growth inhibition assay | Inhibition of human NCI-SNU-5 cell growth in a cell viability assay, IC50 = 0.04585 μM. | SANGER | |||
| TE-8 | Growth inhibition assay | Inhibition of human TE-8 cell growth in a cell viability assay, IC50 = 0.04598 μM. | SANGER | |||
| LB373-MEL-D | Growth inhibition assay | Inhibition of human LB373-MEL-D cell growth in a cell viability assay, IC50 = 0.0468 μM. | SANGER | |||
| PANC-08-13 | Growth inhibition assay | Inhibition of human PANC-08-13 cell growth in a cell viability assay, IC50 = 0.04695 μM. | SANGER | |||
| LU-134-A | Growth inhibition assay | Inhibition of human LU-134-A cell growth in a cell viability assay, IC50 = 0.04722 μM. | SANGER | |||
| COLO-824 | Growth inhibition assay | Inhibition of human COLO-824 cell growth in a cell viability assay, IC50 = 0.04724 μM. | SANGER | |||
| DU-4475 | Growth inhibition assay | Inhibition of human DU-4475 cell growth in a cell viability assay, IC50 = 0.04813 μM. | SANGER | |||
| FADU | Growth inhibition assay | Inhibition of human FADU cell growth in a cell viability assay, IC50 = 0.04852 μM. | SANGER | |||
| NCI-H2405 | Growth inhibition assay | Inhibition of human NCI-H2405 cell growth in a cell viability assay, IC50 = 0.05071 μM. | SANGER | |||
| GCT | Growth inhibition assay | Inhibition of human GCT cell growth in a cell viability assay, IC50 = 0.05077 μM. | SANGER | |||
| NCI-H209 | Growth inhibition assay | Inhibition of human NCI-H209 cell growth in a cell viability assay, IC50 = 0.0508 μM. | SANGER | |||
| HOP-92 | Growth inhibition assay | Inhibition of human HOP-92 cell growth in a cell viability assay, IC50 = 0.0513 μM. | SANGER | |||
| SK-LU-1 | Growth inhibition assay | Inhibition of human SK-LU-1 cell growth in a cell viability assay, IC50 = 0.05183 μM. | SANGER | |||
| LU-139 | Growth inhibition assay | Inhibition of human LU-139 cell growth in a cell viability assay, IC50 = 0.05288 μM. | SANGER | |||
| COLO-680N | Growth inhibition assay | Inhibition of human COLO-680N cell growth in a cell viability assay, IC50 = 0.05331 μM. | SANGER | |||
| NCI-H1395 | Growth inhibition assay | Inhibition of human NCI-H1395 cell growth in a cell viability assay, IC50 = 0.05349 μM. | SANGER | |||
| T84 | Growth inhibition assay | Inhibition of human T84 cell growth in a cell viability assay, IC50 = 0.05386 μM. | SANGER | |||
| NCI-H748 | Growth inhibition assay | Inhibition of human NCI-H748 cell growth in a cell viability assay, IC50 = 0.05695 μM. | SANGER | |||
| HCC2218 | Growth inhibition assay | Inhibition of human HCC2218 cell growth in a cell viability assay, IC50 = 0.05706 μM. | SANGER | |||
| GCIY | Growth inhibition assay | Inhibition of human GCIY cell growth in a cell viability assay, IC50 = 0.05788 μM. | SANGER | |||
| NCI-H1092 | Growth inhibition assay | Inhibition of human NCI-H1092 cell growth in a cell viability assay, IC50 = 0.05807 μM. | SANGER | |||
| BEN | Growth inhibition assay | Inhibition of human BEN cell growth in a cell viability assay, IC50 = 0.05811 μM. | SANGER | |||
| CaR-1 | Growth inhibition assay | Inhibition of human CaR-1 cell growth in a cell viability assay, IC50 = 0.05907 μM. | SANGER | |||
| NCI-H82 | Growth inhibition assay | Inhibition of human NCI-H82 cell growth in a cell viability assay, IC50 = 0.06002 μM. | SANGER | |||
| NCI-H23 | Growth inhibition assay | Inhibition of human NCI-H23 cell growth in a cell viability assay, IC50 = 0.06005 μM. | SANGER | |||
| KMS-12-PE | Growth inhibition assay | Inhibition of human KMS-12-PE cell growth in a cell viability assay, IC50 = 0.06015 μM. | SANGER | |||
| NCI-H1651 | Growth inhibition assay | Inhibition of human NCI-H1651 cell growth in a cell viability assay, IC50 = 0.06064 μM. | SANGER | |||
| NCI-H2052 | Growth inhibition assay | Inhibition of human NCI-H2052 cell growth in a cell viability assay, IC50 = 0.06098 μM. | SANGER | |||
| BB65-RCC | Growth inhibition assay | Inhibition of human BB65-RCC cell growth in a cell viability assay, IC50 = 0.06184 μM. | SANGER | |||
| D-336MG | Growth inhibition assay | Inhibition of human D-336MG cell growth in a cell viability assay, IC50 = 0.06389 μM. | SANGER | |||
| D-392MG | Growth inhibition assay | Inhibition of human D-392MG cell growth in a cell viability assay, IC50 = 0.06593 μM. | SANGER | |||
| NCI-H747 | Growth inhibition assay | Inhibition of human NCI-H747 cell growth in a cell viability assay, IC50 = 0.06719 μM. | SANGER | |||
| KARPAS-299 | Growth inhibition assay | Inhibition of human KARPAS-299 cell growth in a cell viability assay, IC50 = 0.0675 μM. | SANGER | |||
| LOXIMVI | Growth inhibition assay | Inhibition of human LOXIMVI cell growth in a cell viability assay, IC50 = 0.06903 μM. | SANGER | |||
| TGBC1TKB | Growth inhibition assay | Inhibition of human TGBC1TKB cell growth in a cell viability assay, IC50 = 0.0695 μM. | SANGER | |||
| NCI-H524 | Growth inhibition assay | Inhibition of human NCI-H524 cell growth in a cell viability assay, IC50 = 0.06987 μM. | SANGER | |||
| GOTO | Growth inhibition assay | Inhibition of human GOTO cell growth in a cell viability assay, IC50 = 0.07054 μM. | SANGER | |||
| Caov-3 | Growth inhibition assay | Inhibition of human Caov-3 cell growth in a cell viability assay, IC50 = 0.0716 μM. | SANGER | |||
| KINGS-1 | Growth inhibition assay | Inhibition of human KINGS-1 cell growth in a cell viability assay, IC50 = 0.07265 μM. | SANGER | |||
| SW1990 | Growth inhibition assay | Inhibition of human SW1990 cell growth in a cell viability assay, IC50 = 0.07319 μM. | SANGER | |||
| KOSC-2 | Growth inhibition assay | Inhibition of human KOSC-2 cell growth in a cell viability assay, IC50 = 0.07457 μM. | SANGER | |||
| NB5 | Growth inhibition assay | Inhibition of human NB5 cell growth in a cell viability assay, IC50 = 0.07534 μM. | SANGER | |||
| UMC-11 | Growth inhibition assay | Inhibition of human UMC-11 cell growth in a cell viability assay, IC50 = 0.07701 μM. | SANGER | |||
| NCI-H28 | Growth inhibition assay | Inhibition of human NCI-H28 cell growth in a cell viability assay, IC50 = 0.07875 μM. | SANGER | |||
| TGW | Growth inhibition assay | Inhibition of human TGW cell growth in a cell viability assay, IC50 = 0.0789 μM. | SANGER | |||
| SW1088 | Growth inhibition assay | Inhibition of human SW1088 cell growth in a cell viability assay, IC50 = 0.07944 μM. | SANGER | |||
| MS-1 | Growth inhibition assay | Inhibition of human MS-1 cell growth in a cell viability assay, IC50 = 0.07996 μM. | SANGER | |||
| Raji | Growth inhibition assay | Inhibition of human Raji cell growth in a cell viability assay, IC50 = 0.08164 μM. | SANGER | |||
| DG-75 | Growth inhibition assay | Inhibition of human DG-75 cell growth in a cell viability assay, IC50 = 0.08204 μM. | SANGER | |||
| D-502MG | Growth inhibition assay | Inhibition of human D-502MG cell growth in a cell viability assay, IC50 = 0.08263 μM. | SANGER | |||
| D-542MG | Growth inhibition assay | Inhibition of human D-542MG cell growth in a cell viability assay, IC50 = 0.08331 μM. | SANGER | |||
| Daudi | Growth inhibition assay | Inhibition of human Daudi cell growth in a cell viability assay, IC50 = 0.08492 μM. | SANGER | |||
| LB1047-RCC | Growth inhibition assay | Inhibition of human LB1047-RCC cell growth in a cell viability assay, IC50 = 0.08558 μM. | SANGER | |||
| TE-6 | Growth inhibition assay | Inhibition of human TE-6 cell growth in a cell viability assay, IC50 = 0.0872 μM. | SANGER | |||
| EHEB | Growth inhibition assay | Inhibition of human EHEB cell growth in a cell viability assay, IC50 = 0.09037 μM. | SANGER | |||
| RMG-I | Growth inhibition assay | Inhibition of human RMG-I cell growth in a cell viability assay, IC50 = 0.09379 μM. | SANGER | |||
| JAR | Growth inhibition assay | Inhibition of human JAR cell growth in a cell viability assay, IC50 = 0.0942 μM. | SANGER | |||
| NCI-H1792 | Growth inhibition assay | Inhibition of human NCI-H1792 cell growth in a cell viability assay, IC50 = 0.09513 μM. | SANGER | |||
| CHP-126 | Growth inhibition assay | Inhibition of human CHP-126 cell growth in a cell viability assay, IC50 = 0.09679 μM. | SANGER | |||
| J82 | Growth inhibition assay | Inhibition of human J82 cell growth in a cell viability assay, IC50 = 0.09779 μM. | SANGER | |||
| SNU-C1 | Growth inhibition assay | Inhibition of human SNU-C1 cell growth in a cell viability assay, IC50 = 0.09797 μM. | SANGER | |||
| MDA-MB-157 | Growth inhibition assay | Inhibition of human MDA-MB-157 cell growth in a cell viability assay, IC50 = 0.09878 μM. | SANGER | |||
| PLC-PRF-5 | Growth inhibition assay | Inhibition of human PLC-PRF-5 cell growth in a cell viability assay, IC50 = 0.1017 μM. | SANGER | |||
| C2BBe1 | Growth inhibition assay | Inhibition of human C2BBe1 cell growth in a cell viability assay, IC50 = 0.10183 μM. | SANGER | |||
| GAK | Growth inhibition assay | Inhibition of human GAK cell growth in a cell viability assay, IC50 = 0.10272 μM. | SANGER | |||
| PC-3 | Growth inhibition assay | Inhibition of human PC-3 cell growth in a cell viability assay, IC50 = 0.10448 μM. | SANGER | |||
| KG-1 | Growth inhibition assay | Inhibition of human KG-1 cell growth in a cell viability assay, IC50 = 0.10505 μM. | SANGER | |||
| L-428 | Growth inhibition assay | Inhibition of human L-428 cell growth in a cell viability assay, IC50 = 0.10562 μM. | SANGER | |||
| HL-60 | Growth inhibition assay | Inhibition of human HL-60 cell growth in a cell viability assay, IC50 = 0.10636 μM. | SANGER | |||
| WM-115 | Growth inhibition assay | Inhibition of human WM-115 cell growth in a cell viability assay, IC50 = 0.10834 μM. | SANGER | |||
| NCI-H2171 | Growth inhibition assay | Inhibition of human NCI-H2171 cell growth in a cell viability assay, IC50 = 0.10928 μM. | SANGER | |||
| OVCAR-5 | Growth inhibition assay | Inhibition of human OVCAR-5 cell growth in a cell viability assay, IC50 = 0.11016 μM. | SANGER | |||
| TYK-nu | Growth inhibition assay | Inhibition of human TYK-nu cell growth in a cell viability assay, IC50 = 0.11109 μM. | SANGER | |||
| SIMA | Growth inhibition assay | Inhibition of human SIMA cell growth in a cell viability assay, IC50 = 0.11296 μM. | SANGER | |||
| SNU-475 | Growth inhibition assay | Inhibition of human SNU-475 cell growth in a cell viability assay, IC50 = 0.11425 μM. | SANGER | |||
| NB10 | Growth inhibition assay | Inhibition of human NB10 cell growth in a cell viability assay, IC50 = 0.11454 μM. | SANGER | |||
| DOK | Growth inhibition assay | Inhibition of human DOK cell growth in a cell viability assay, IC50 = 0.11581 μM. | SANGER | |||
| TK10 | Growth inhibition assay | Inhibition of human TK10 cell growth in a cell viability assay, IC50 = 0.11625 μM. | SANGER | |||
| HCC1937 | Growth inhibition assay | Inhibition of human HCC1937 cell growth in a cell viability assay, IC50 = 0.11742 μM. | SANGER | |||
| COR-L279 | Growth inhibition assay | Inhibition of human COR-L279 cell growth in a cell viability assay, IC50 = 0.11861 μM. | SANGER | |||
| U-118-MG | Growth inhibition assay | Inhibition of human U-118-MG cell growth in a cell viability assay, IC50 = 0.12052 μM. | SANGER | |||
| EC-GI-10 | Growth inhibition assay | Inhibition of human EC-GI-10 cell growth in a cell viability assay, IC50 = 0.12227 μM. | SANGER | |||
| THP-1 | Growth inhibition assay | Inhibition of human THP-1 cell growth in a cell viability assay, IC50 = 0.12495 μM. | SANGER | |||
| NB6 | Growth inhibition assay | Inhibition of human NB6 cell growth in a cell viability assay, IC50 = 0.12568 μM. | SANGER | |||
| CAL-54 | Growth inhibition assay | Inhibition of human CAL-54 cell growth in a cell viability assay, IC50 = 0.12613 μM. | SANGER | |||
| HT-3 | Growth inhibition assay | Inhibition of human HT-3 cell growth in a cell viability assay, IC50 = 0.12829 μM. | SANGER | |||
| GMS-10 | Growth inhibition assay | Inhibition of human GMS-10 cell growth in a cell viability assay, IC50 = 0.1289 μM. | SANGER | |||
| EGI-1 | Growth inhibition assay | Inhibition of human EGI-1 cell growth in a cell viability assay, IC50 = 0.12994 μM. | SANGER | |||
| HT-1197 | Growth inhibition assay | Inhibition of human HT-1197 cell growth in a cell viability assay, IC50 = 0.1324 μM. | SANGER | |||
| KMOE-2 | Growth inhibition assay | Inhibition of human KMOE-2 cell growth in a cell viability assay, IC50 = 0.13623 μM. | SANGER | |||
| MFE-280 | Growth inhibition assay | Inhibition of human MFE-280 cell growth in a cell viability assay, IC50 = 0.14433 μM. | SANGER | |||
| ES4 | Growth inhibition assay | Inhibition of human ES4 cell growth in a cell viability assay, IC50 = 0.14614 μM. | SANGER | |||
| SW756 | Growth inhibition assay | Inhibition of human SW756 cell growth in a cell viability assay, IC50 = 0.14838 μM. | SANGER | |||
| SKG-IIIa | Growth inhibition assay | Inhibition of human SKG-IIIa cell growth in a cell viability assay, IC50 = 0.15554 μM. | SANGER | |||
| IST-MEL1 | Growth inhibition assay | Inhibition of human IST-MEL1 cell growth in a cell viability assay, IC50 = 0.15589 μM. | SANGER | |||
| no-11 | Growth inhibition assay | Inhibition of human no-11 cell growth in a cell viability assay, IC50 = 0.16348 μM. | SANGER | |||
| MN-60 | Growth inhibition assay | Inhibition of human MN-60 cell growth in a cell viability assay, IC50 = 0.1676 μM. | SANGER | |||
| SW1417 | Growth inhibition assay | Inhibition of human SW1417 cell growth in a cell viability assay, IC50 = 0.17327 μM. | SANGER | |||
| KLE | Growth inhibition assay | Inhibition of human KLE cell growth in a cell viability assay, IC50 = 0.17367 μM. | SANGER | |||
| SNU-449 | Growth inhibition assay | Inhibition of human SNU-449 cell growth in a cell viability assay, IC50 = 0.1738 μM. | SANGER | |||
| EW-3 | Growth inhibition assay | Inhibition of human EW-3 cell growth in a cell viability assay, IC50 = 0.18223 μM. | SANGER | |||
| NCI-H1522 | Growth inhibition assay | Inhibition of human NCI-H1522 cell growth in a cell viability assay, IC50 = 0.18527 μM. | SANGER | |||
| COLO-678 | Growth inhibition assay | Inhibition of human COLO-678 cell growth in a cell viability assay, IC50 = 0.18905 μM. | SANGER | |||
| ECC4 | Growth inhibition assay | Inhibition of human ECC4 cell growth in a cell viability assay, IC50 = 0.18919 μM. | SANGER | |||
| GB-1 | Growth inhibition assay | Inhibition of human GB-1 cell growth in a cell viability assay, IC50 = 0.18959 μM. | SANGER | |||
| BOKU | Growth inhibition assay | Inhibition of human BOKU cell growth in a cell viability assay, IC50 = 0.19522 μM. | SANGER | |||
| LU-165 | Growth inhibition assay | Inhibition of human LU-165 cell growth in a cell viability assay, IC50 = 0.19534 μM. | SANGER | |||
| MDA-MB-453 | Growth inhibition assay | Inhibition of human MDA-MB-453 cell growth in a cell viability assay, IC50 = 0.19951 μM. | SANGER | |||
| C3A | Growth inhibition assay | Inhibition of human C3A cell growth in a cell viability assay, IC50 = 0.20959 μM. | SANGER | |||
| BB49-HNC | Growth inhibition assay | Inhibition of human BB49-HNC cell growth in a cell viability assay, IC50 = 0.20972 μM. | SANGER | |||
| LB2518-MEL | Growth inhibition assay | Inhibition of human LB2518-MEL cell growth in a cell viability assay, IC50 = 0.21062 μM. | SANGER | |||
| LS-1034 | Growth inhibition assay | Inhibition of human LS-1034 cell growth in a cell viability assay, IC50 = 0.21618 μM. | SANGER | |||
| YT | Growth inhibition assay | Inhibition of human YT cell growth in a cell viability assay, IC50 = 0.21854 μM. | SANGER | |||
| COLO-792 | Growth inhibition assay | Inhibition of human COLO-792 cell growth in a cell viability assay, IC50 = 0.22044 μM. | SANGER | |||
| NCI-H727 | Growth inhibition assay | Inhibition of human NCI-H727 cell growth in a cell viability assay, IC50 = 0.22454 μM. | SANGER | |||
| MHH-CALL-2 | Growth inhibition assay | Inhibition of human MHH-CALL-2 cell growth in a cell viability assay, IC50 = 0.24193 μM. | SANGER | |||
| LN-405 | Growth inhibition assay | Inhibition of human LN-405 cell growth in a cell viability assay, IC50 = 0.24504 μM. | SANGER | |||
| SK-N-DZ | Growth inhibition assay | Inhibition of human SK-N-DZ cell growth in a cell viability assay, IC50 = 0.24904 μM. | SANGER | |||
| HuP-T3 | Growth inhibition assay | Inhibition of human HuP-T3 cell growth in a cell viability assay, IC50 = 0.25614 μM. | SANGER | |||
| MKN7 | Growth inhibition assay | Inhibition of human MKN7 cell growth in a cell viability assay, IC50 = 0.25838 μM. | SANGER | |||
| EW-24 | Growth inhibition assay | Inhibition of human EW-24 cell growth in a cell viability assay, IC50 = 0.27474 μM. | SANGER | |||
| EVSA-T | Growth inhibition assay | Inhibition of human EVSA-T cell growth in a cell viability assay, IC50 = 0.27519 μM. | SANGER | |||
| MDA-MB-175-VII | Growth inhibition assay | Inhibition of human MDA-MB-175-VII cell growth in a cell viability assay, IC50 = 0.28065 μM. | SANGER | |||
| SiHa | Growth inhibition assay | Inhibition of human SiHa cell growth in a cell viability assay, IC50 = 0.28127 μM. | SANGER | |||
| T-24 | Growth inhibition assay | Inhibition of human T-24 cell growth in a cell viability assay, IC50 = 0.28425 μM. | SANGER | |||
| EKVX | Growth inhibition assay | Inhibition of human EKVX cell growth in a cell viability assay, IC50 = 0.28708 μM. | SANGER | |||
| JVM-3 | Growth inhibition assay | Inhibition of human JVM-3 cell growth in a cell viability assay, IC50 = 0.29206 μM. | SANGER | |||
| MDA-MB-134-VI | Growth inhibition assay | Inhibition of human MDA-MB-134-VI cell growth in a cell viability assay, IC50 = 0.30194 μM. | SANGER | |||
| LB996-RCC | Growth inhibition assay | Inhibition of human LB996-RCC cell growth in a cell viability assay, IC50 = 0.30883 μM. | SANGER | |||
| FTC-133 | Growth inhibition assay | Inhibition of human FTC-133 cell growth in a cell viability assay, IC50 = 0.30939 μM. | SANGER | |||
| LP-1 | Growth inhibition assay | Inhibition of human LP-1 cell growth in a cell viability assay, IC50 = 0.31132 μM. | SANGER | |||
| EFE-184 | Growth inhibition assay | Inhibition of human EFE-184 cell growth in a cell viability assay, IC50 = 0.31668 μM. | SANGER | |||
| CPC-N | Growth inhibition assay | Inhibition of human CPC-N cell growth in a cell viability assay, IC50 = 0.31876 μM. | SANGER | |||
| NCI-H2196 | Growth inhibition assay | Inhibition of human NCI-H2196 cell growth in a cell viability assay, IC50 = 0.32849 μM. | SANGER | |||
| RT4 | Growth inhibition assay | Inhibition of human RT4 cell growth in a cell viability assay, IC50 = 0.33062 μM. | SANGER | |||
| MPP-89 | Growth inhibition assay | Inhibition of human MPP-89 cell growth in a cell viability assay, IC50 = 0.34294 μM. | SANGER | |||
| DMS-153 | Growth inhibition assay | Inhibition of human DMS-153 cell growth in a cell viability assay, IC50 = 0.35024 μM. | SANGER | |||
| LB2241-RCC | Growth inhibition assay | Inhibition of human LB2241-RCC cell growth in a cell viability assay, IC50 = 0.35241 μM. | SANGER | |||
| S-117 | Growth inhibition assay | Inhibition of human S-117 cell growth in a cell viability assay, IC50 = 0.36822 μM. | SANGER | |||
| SW837 | Growth inhibition assay | Inhibition of human SW837 cell growth in a cell viability assay, IC50 = 0.37965 μM. | SANGER | |||
| HCC1419 | Growth inhibition assay | Inhibition of human HCC1419 cell growth in a cell viability assay, IC50 = 0.39656 μM. | SANGER | |||
| T47D | Growth inhibition assay | Inhibition of human T47D cell growth in a cell viability assay, IC50 = 0.40041 μM. | SANGER | |||
| DMS-114 | Growth inhibition assay | Inhibition of human DMS-114 cell growth in a cell viability assay, IC50 = 0.40102 μM. | SANGER | |||
| SW1463 | Growth inhibition assay | Inhibition of human SW1463 cell growth in a cell viability assay, IC50 = 0.40946 μM. | SANGER | |||
| RL | Growth inhibition assay | Inhibition of human RL cell growth in a cell viability assay, IC50 = 0.41513 μM. | SANGER | |||
| HCC1143 | Growth inhibition assay | Inhibition of human HCC1143 cell growth in a cell viability assay, IC50 = 0.43816 μM. | SANGER | |||
| EB-3 | Growth inhibition assay | Inhibition of human EB-3 cell growth in a cell viability assay, IC50 = 0.44051 μM. | SANGER | |||
| SF126 | Growth inhibition assay | Inhibition of human SF126 cell growth in a cell viability assay, IC50 = 0.45923 μM. | SANGER | |||
| KY821 | Growth inhibition assay | Inhibition of human KY821 cell growth in a cell viability assay, IC50 = 0.47276 μM. | SANGER | |||
| LS-513 | Growth inhibition assay | Inhibition of human LS-513 cell growth in a cell viability assay, IC50 = 0.48288 μM. | SANGER | |||
| SW1783 | Growth inhibition assay | Inhibition of human SW1783 cell growth in a cell viability assay, IC50 = 0.49239 μM. | SANGER | |||
| TE-12 | Growth inhibition assay | Inhibition of human TE-12 cell growth in a cell viability assay, IC50 = 0.49653 μM. | SANGER | |||
| D-263MG | Growth inhibition assay | Inhibition of human D-263MG cell growth in a cell viability assay, IC50 = 0.50551 μM. | SANGER | |||
| SHP-77 | Growth inhibition assay | Inhibition of human SHP-77 cell growth in a cell viability assay, IC50 = 0.50808 μM. | SANGER | |||
| BHT-101 | Growth inhibition assay | Inhibition of human BHT-101 cell growth in a cell viability assay, IC50 = 0.51311 μM. | SANGER | |||
| Calu-1 | Growth inhibition assay | Inhibition of human Calu-1 cell growth in a cell viability assay, IC50 = 0.51639 μM. | SANGER | |||
| COLO-320-HSR | Growth inhibition assay | Inhibition of human COLO-320-HSR cell growth in a cell viability assay, IC50 = 0.52165 μM. | SANGER | |||
| IST-MES1 | Growth inhibition assay | Inhibition of human IST-MES1 cell growth in a cell viability assay, IC50 = 0.53233 μM. | SANGER | |||
| DMS-79 | Growth inhibition assay | Inhibition of human DMS-79 cell growth in a cell viability assay, IC50 = 0.53781 μM. | SANGER | |||
| IM-9 | Growth inhibition assay | Inhibition of human IM-9 cell growth in a cell viability assay, IC50 = 0.54557 μM. | SANGER | |||
| SK-MEL-28 | Growth inhibition assay | Inhibition of human SK-MEL-28 cell growth in a cell viability assay, IC50 = 0.61539 μM. | SANGER | |||
| CAL-72 | Growth inhibition assay | Inhibition of human CAL-72 cell growth in a cell viability assay, IC50 = 0.61825 μM. | SANGER | |||
| HSC-4 | Growth inhibition assay | Inhibition of human HSC-4 cell growth in a cell viability assay, IC50 = 0.62725 μM. | SANGER | |||
| D-283MED | Growth inhibition assay | Inhibition of human D-283MED cell growth in a cell viability assay, IC50 = 0.64284 μM. | SANGER | |||
| NCI-H596 | Growth inhibition assay | Inhibition of human NCI-H596 cell growth in a cell viability assay, IC50 = 0.65934 μM. | SANGER | |||
| NCI-H1618 | Growth inhibition assay | Inhibition of human NCI-H1618 cell growth in a cell viability assay, IC50 = 0.71488 μM. | SANGER | |||
| NCI-H1993 | Growth inhibition assay | Inhibition of human NCI-H1993 cell growth in a cell viability assay, IC50 = 0.74088 μM. | SANGER | |||
| RCM-1 | Growth inhibition assay | Inhibition of human RCM-1 cell growth in a cell viability assay, IC50 = 0.7478 μM. | SANGER | |||
| KNS-81-FD | Growth inhibition assay | Inhibition of human KNS-81-FD cell growth in a cell viability assay, IC50 = 0.8079 μM. | SANGER | |||
| SK-N-AS | Growth inhibition assay | Inhibition of human SK-N-AS cell growth in a cell viability assay, IC50 = 0.86756 μM. | SANGER | |||
| SCLC-21H | Growth inhibition assay | Inhibition of human SCLC-21H cell growth in a cell viability assay, IC50 = 0.90851 μM. | SANGER | |||
| DMS-53 | Growth inhibition assay | Inhibition of human DMS-53 cell growth in a cell viability assay, IC50 = 0.91644 μM. | SANGER | |||
| UACC-893 | Growth inhibition assay | Inhibition of human UACC-893 cell growth in a cell viability assay, IC50 = 1.09233 μM. | SANGER | |||
| KU-19-19 | Growth inhibition assay | Inhibition of human KU-19-19 cell growth in a cell viability assay, IC50 = 1.11505 μM. | SANGER | |||
| C32 | Growth inhibition assay | Inhibition of human C32 cell growth in a cell viability assay, IC50 = 1.11824 μM. | SANGER | |||
| HCE-T | Growth inhibition assay | Inhibition of human HCE-T cell growth in a cell viability assay, IC50 = 1.15381 μM. | SANGER | |||
| Saos-2 | Growth inhibition assay | Inhibition of human Saos-2 cell growth in a cell viability assay, IC50 = 1.17363 μM. | SANGER | |||
| NCI-H2227 | Growth inhibition assay | Inhibition of human NCI-H2227 cell growth in a cell viability assay, IC50 = 1.18132 μM. | SANGER | |||
| Caov-4 | Growth inhibition assay | Inhibition of human Caov-4 cell growth in a cell viability assay, IC50 = 1.22245 μM. | SANGER | |||
| HCT-15 | Growth inhibition assay | Inhibition of human HCT-15 cell growth in a cell viability assay, IC50 = 1.22304 μM. | SANGER | |||
| OMC-1 | Growth inhibition assay | Inhibition of human OMC-1 cell growth in a cell viability assay, IC50 = 1.26488 μM. | SANGER | |||
| CAKI-1 | Growth inhibition assay | Inhibition of human CAKI-1 cell growth in a cell viability assay, IC50 = 1.27128 μM. | SANGER | |||
| YAPC | Growth inhibition assay | Inhibition of human YAPC cell growth in a cell viability assay, IC50 = 1.28878 μM. | SANGER | |||
| SK-MM-2 | Growth inhibition assay | Inhibition of human SK-MM-2 cell growth in a cell viability assay, IC50 = 1.34922 μM. | SANGER | |||
| AsPC-1 | Growth inhibition assay | Inhibition of human AsPC-1 cell growth in a cell viability assay, IC50 = 1.36038 μM. | SANGER | |||
| RO82-W-1 | Growth inhibition assay | Inhibition of human RO82-W-1 cell growth in a cell viability assay, IC50 = 1.37101 μM. | SANGER | |||
| LAN-6 | Growth inhibition assay | Inhibition of human LAN-6 cell growth in a cell viability assay, IC50 = 1.42458 μM. | SANGER | |||
| HN | Growth inhibition assay | Inhibition of human HN cell growth in a cell viability assay, IC50 = 1.43413 μM. | SANGER | |||
| SK-MEL-1 | Growth inhibition assay | Inhibition of human SK-MEL-1 cell growth in a cell viability assay, IC50 = 1.51819 μM. | SANGER | |||
| U031 | Growth inhibition assay | Inhibition of human U031 cell growth in a cell viability assay, IC50 = 1.55775 μM. | SANGER | |||
| HT55 | Growth inhibition assay | Inhibition of human HT55 cell growth in a cell viability assay, IC50 = 1.72747 μM. | SANGER | |||
| SCC-3 | Growth inhibition assay | Inhibition of human SCC-3 cell growth in a cell viability assay, IC50 = 1.7531 μM. | SANGER | |||
| SK-MEL-24 | Growth inhibition assay | Inhibition of human SK-MEL-24 cell growth in a cell viability assay, IC50 = 1.7671 μM. | SANGER | |||
| NCI-H2452 | Growth inhibition assay | Inhibition of human NCI-H2452 cell growth in a cell viability assay, IC50 = 2.0176 μM. | SANGER | |||
| TT | Growth inhibition assay | Inhibition of human TT cell growth in a cell viability assay, IC50 = 2.27099 μM. | SANGER | |||
| LS-123 | Growth inhibition assay | Inhibition of human LS-123 cell growth in a cell viability assay, IC50 = 2.33753 μM. | SANGER | |||
| Hs-578-T | Growth inhibition assay | Inhibition of human Hs-578-T cell growth in a cell viability assay, IC50 = 2.45525 μM. | SANGER | |||
| SBC-1 | Growth inhibition assay | Inhibition of human SBC-1 cell growth in a cell viability assay, IC50 = 2.56462 μM. | SANGER | |||
| SCH | Growth inhibition assay | Inhibition of human SCH cell growth in a cell viability assay, IC50 = 3.18837 μM. | SANGER | |||
| RCC10RGB | Growth inhibition assay | Inhibition of human RCC10RGB cell growth in a cell viability assay, IC50 = 3.29613 μM. | SANGER | |||
| K-562 | Growth inhibition assay | Inhibition of human K-562 cell growth in a cell viability assay, IC50 = 3.29943 μM. | SANGER | |||
| SK-N-FI | Growth inhibition assay | Inhibition of human SK-N-FI cell growth in a cell viability assay, IC50 = 3.72776 μM. | SANGER | |||
| HAL-01 | Growth inhibition assay | Inhibition of human HAL-01 cell growth in a cell viability assay, IC50 = 3.94137 μM. | SANGER | |||
| ES5 | Growth inhibition assay | Inhibition of human ES5 cell growth in a cell viability assay, IC50 = 4.15835 μM. | SANGER | |||
| MFM-223 | Growth inhibition assay | Inhibition of human MFM-223 cell growth in a cell viability assay, IC50 = 4.20908 μM. | SANGER | |||
| NCI-H716 | Growth inhibition assay | Inhibition of human NCI-H716 cell growth in a cell viability assay, IC50 = 4.37889 μM. | SANGER | |||
| SAS | Growth inhibition assay | Inhibition of human SAS cell growth in a cell viability assay, IC50 = 4.39125 μM. | SANGER | |||
| PSN1 | Growth inhibition assay | Inhibition of human PSN1 cell growth in a cell viability assay, IC50 = 4.44423 μM. | SANGER | |||
| A704 | Growth inhibition assay | Inhibition of human A704 cell growth in a cell viability assay, IC50 = 5.84137 μM. | SANGER | |||
| NCI-H719 | Growth inhibition assay | Inhibition of human NCI-H719 cell growth in a cell viability assay, IC50 = 5.90333 μM. | SANGER | |||
| U-2-OS | Growth inhibition assay | Inhibition of human U-2-OS cell growth in a cell viability assay, IC50 = 6.30268 μM. | SANGER | |||
| NCI-H322M | Growth inhibition assay | Inhibition of human NCI-H322M cell growth in a cell viability assay, IC50 = 6.40749 μM. | SANGER | |||
| DK-MG | Growth inhibition assay | Inhibition of human DK-MG cell growth in a cell viability assay, IC50 = 6.44516 μM. | SANGER | |||
| NCI-H1693 | Growth inhibition assay | Inhibition of human NCI-H1693 cell growth in a cell viability assay, IC50 = 7.89571 μM. | SANGER | |||
| JVM-2 | Growth inhibition assay | Inhibition of human JVM-2 cell growth in a cell viability assay, IC50 = 8.39779 μM. | SANGER | |||
| MSTO-211H | Growth inhibition assay | Inhibition of human MSTO-211H cell growth in a cell viability assay, IC50 = 8.80042 μM. | SANGER | |||
| UACC-812 | Growth inhibition assay | Inhibition of human UACC-812 cell growth in a cell viability assay, IC50 = 8.91355 μM. | SANGER | |||
| CW-2 | Growth inhibition assay | Inhibition of human CW-2 cell growth in a cell viability assay, IC50 = 9.32494 μM. | SANGER | |||
| NCI-H1155 | Growth inhibition assay | Inhibition of human NCI-H1155 cell growth in a cell viability assay, IC50 = 9.63085 μM. | SANGER | |||
| NCI-H720 | Growth inhibition assay | Inhibition of human NCI-H720 cell growth in a cell viability assay, IC50 = 10.9542 μM. | SANGER | |||
| P31-FUJ | Growth inhibition assay | Inhibition of human P31-FUJ cell growth in a cell viability assay, IC50 = 11.3858 μM. | SANGER | |||
| TALL-1 | Growth inhibition assay | Inhibition of human TALL-1 cell growth in a cell viability assay, IC50 = 11.8714 μM. | SANGER | |||
| NCI-H1838 | Growth inhibition assay | Inhibition of human NCI-H1838 cell growth in a cell viability assay, IC50 = 12.2348 μM. | SANGER | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(92.66 mM)
Water : 100 mg/mL Ethanol : 100 mg/mL |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 1079.11 | Formula | C45H54N4O8.2C4H6O6 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 125317-39-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | KW-2307 ditartrate | Smiles | CCC1=CC2CC(C3=C(CN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7C(C=CC9)(C(C(C8N6C)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC.C(C(C(=O)O)O)(C(=O)O)O.C(C(C(=O)O)O)(C(=O)O)O | ||
Mechanism of Action
| Features |
A semi-synthetic vinca alkaloid as an anti-mitotic chemotherapy drug.
|
|---|---|
| Targets/IC50/Ki |
Tubulin
(Cell-free assay) |
| In vitro |
Vinorelbine inhibits microtubule assembly by inducing tubulin aggregation into spirals and paracrystals. Vinorelbine shows potent antiproliferative activity against a series of tumor cells, including human melanoma, non-small-cell lung cancer, breast cancer, etc. |
| In vivo |
In vivo, Vinorelbine also shows antitumour activity against a series of subcutaneously-implanted human tumour xenografts. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | Cyclin A / Cyclin B1 / p-Histone H3 / CDK1 / PCNA |
|
19017359 |
| Immunofluorescence | RPS6 / G3BP1 / TIAR G3BP1 / eIF3b / eIF4G |
|
27083003 |
| Growth inhibition assay | Cell number |
|
19017359 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04896320 | Withdrawn | Breast Cancer Stage IV |
Providence Health & Services|Seagen Inc. |
December 15 2021 | Phase 1|Phase 2 |
| NCT04299113 | Recruiting | Rhabdomyosarcoma |
Jonsson Comprehensive Cancer Center|Mirati Therapeutics Inc.|Phase One Foundation |
May 14 2020 | Phase 1 |
| NCT03891173 | Terminated | Lung Adenocarcinoma |
Helix BioPharma Corporation|KCR S.A. |
February 19 2019 | Phase 2 |
| NCT02925000 | Completed | Cancer |
Taiwan Liposome Company |
June 19 2017 | Phase 1|Phase 2 |
| NCT02658084 | Terminated | Breast Cancer|Metastatic Breast Cancer |
University of Miami|Genentech Inc. |
April 12 2017 | Phase 1|Phase 2 |
| NCT02768415 | Unknown status | Breast Cancer |
Chinese Academy of Medical Sciences|ChineseAMS |
June 2016 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































