research use only
Epothilone A Microtubule Associated inhibitor
Cat.No.S1297
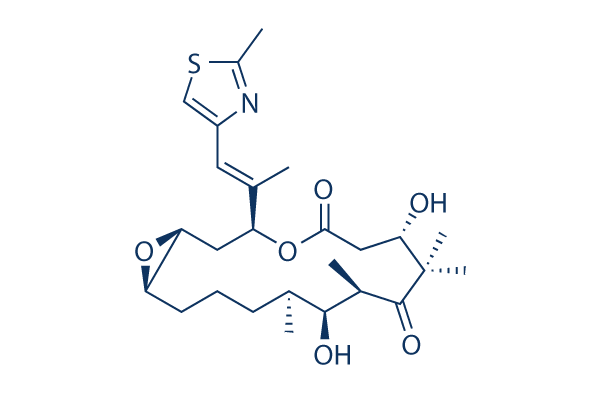
Chemical Structure
Molecular Weight: 493.66
Quality Control
| Related Targets | Akt Wnt/beta-catenin PKC HSP ROCK Integrin Bcr-Abl Actin FAK Kinesin |
|---|---|
| Other Microtubule Associated Inhibitors | Nocodazole MMAF Patupilone (Epothilone B) CW069 Lexibulin (CYT997) Combretastatin A4 ABT-751 (E7010) Cucurbitacin B TAI-1 Vindesine sulfate |
Solubility
|
In vitro |
DMSO
: 99 mg/mL
(200.54 mM)
Ethanol : 99 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 493.66 | Formula | C26H39NO6S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 152044-53-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CC1CCCC2C(O2)CC(OC(=O)CC(C(C(=O)C(C1O)C)(C)C)O)C(=CC3=CSC(=N3)C)C | ||
Mechanism of Action
| Targets/IC50/Ki |
Tubulin
2 μM(EC0.01)
|
|---|---|
| In vitro |
Epothilone A, discovered from the myxobacterium Sorangium cellulosum, is a Taxol-like microtubule-stabilizing agent that induces tubulin polymerization, leading to cell cycle arrest at the G2-M transition, cytotoxicity, and apoptosis.
This compound potently inhibits cell proliferation in HCT116 cells, with IC50 of 4.4 nM.
It also displays cytotoxicity in KB3-1, KBV-1, Hela, and Hs578T cells, with IC50 values ranging from 13 nM to 160 nM. This chemical is more water soluble than Taxol and competes with Taxol in binding with microtubules, with IC50 of 2.3 μM.
However, both this compound and Taxol don't share a common pharmacophore and exploits the tubulin-binding pocket uniquely and independently.
Recently, it is found that microbiological transformation of this compound by Aspergillus niger AS 3.739 yields several metabolites that is also toxic to MCF-7 cells, but with much higher IC50 values.
|
| Kinase Assay |
Tubulin polymerization assay
|
|
Calf brain microtubule proteins (MTP) are purified, which includes approximately 15%–20% microtubule associated proteins. The buffer (MES buffer) used for the Epothilone A-microtubule studies contains 0.1 M 2-morpholinoethanesulfonic acid (MES), 1 mM EGTA, 0.5 mM MgCl2, and 3 M glycerol at pH 6.6. Samples for electron microscopy are placed on carbon-over-Parlodion-coated grids (300 mesh) and negatively stained with 2% uranyl acetate. Microtubule assembly in the presence or absence of this compound is monitored spectrophotometrically by using a spectrophotometer equipped with a thermostatically regulated liquid circulator. The temperature is held at 35 °C and changes in turbidity (representative of polymer mass) are monitored at 350 nm. Effective concentration (EC0.01), defined as the interpolated concentration capable of inducing an initial slope of 0.01 OD/min rate, is calculated using the formula EC0.01 = concentration/slope and expressed as the mean with standard deviation obtained from three different concentrations.
|
References |
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































