research use only
Flubendazole Autophagy activator
Cat.No.S1837
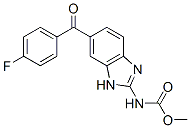
Chemical Structure
Molecular Weight: 313.28
Quality Control
| Related Targets | CXCR Nrf2 Mitophagy LRRK2 ULK FKBP Heme Oxygenase cGAS LC3 Cell wall |
|---|---|
| Other Autophagy Inhibitors | Resveratrol (trans-Resveratrol) Spautin-1 PIK-III DC661 Lys05 Trihydrochloride Autophinib Spermidine SMER28 EN6 QX77 |
Solubility
|
In vitro |
DMSO
: 15 mg/mL
(47.88 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 313.28 | Formula | C16H12FN3O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 31430-15-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Flumoxanal, NSC 313680 | Smiles | COC(=O)NC1=NC2=C(N1)C=C(C=C2)C(=O)C3=CC=C(C=C3)F | ||
Mechanism of Action
| Targets/IC50/Ki |
Atg4B
|
|---|---|
| In vitro |
Flubendazole results in morphological changes included contraction of the soma region, formation of blebs on the tegument, rostellar disorganization, loss of hooks and destruction of microtriches in Echinococcus granulosus. This compound have a bicyclic ring system in which a benzene has been fused to the -4 and -5 positions of the heterocycle (imidazole). It and Albendazole shows similar potency in affecting rat embryonic development in vitro, inducing retardation of growth and dysmorphogenic effects at concentrations ≥0.5 μg/mL.
|
| In vivo |
Flubendazole (6.32 mg/kg/day) initially induces an arrest of embryonic development followed by a generalized cell death that leads to 100% embryolethality by gestation day (GD) 12.5. This compound (3.46 mg/kg/day) markedly reduces embryonic development by GD 12.5 without causing cell death. This chemical in olive oil causes a statistically significant increase in embryolethality at doses of 7.83 mg/kg per day and higher, with complete resorption in all dams at 31.33 mg/kg per day in rats. This treatment causes a slight increase of metyrapone and daunorubicin activities in hepatic as well as intestinal cytosol in birds. It also leads to statistically significant inhibition of intestinal GST activity. The treatment leads to slight but significant inhibition (decrease to 69%) of 7-ethoxyresorufin activity in hepatic microsomes.
|
References |
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































