research use only
Itraconazole Hedgehog/Smoothened inhibitor
Cat.No.S2476
Chemical Structure
Molecular Weight: 705.65031
Quality Control
| Related Targets | JAK TGF-beta/Smad Wnt/beta-catenin ERK GSK-3 ROCK PKA Secretase STAT Casein Kinase |
|---|---|
| Other Hedgehog/Smoothened Inhibitors | SAG (Smoothened Agonist) Hydrochloride Purmorphamine Cyclopamine (11-Deoxojervine) GANT61 SAG (Smoothened Agonist) SANT-1 HPI-4 (Ciliobrevin A) BMS-833923 Taladegib (LY2940680) Ciliobrevin D |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| human hepatocytes | Function assay | Inhibition of CYP3A4 in human hepatocytes using testosterone as substrate by HPLC/MS/MS method, IC50=0.07 μM | ||||
| human HTLA cells | Function assay | 20 mins | Inhibition of CX3CL1-stimulated CX3CR1 in human HTLA cells pre-incubated for 20 mins measured on day 4 by beta arrestin-recruitment mediated luciferase reporter gene assay, IC50=0.1 μM | |||
| LLC-PK1 epithelial cells | Function assay | Inhibition of P-glycoprotein, mouse L-mdr1a expressed in LLC-PK1 epithelial cells using calcein-AM polarisation assay, IC50=0.2 μM | ||||
| human PBMC | Cytotoxicity assay | 72 h | Cytotoxicity against human PBMC assessed as cell viability after 72 hrs by MTT assay, IC50=1.53 μM | |||
| Topp 3 cells | Function assay | Inhibition of human CYP51 expressed in Topp 3 cells by lanosterol demethylase assay, IC50=3.6 μM | ||||
| human MRC5 cells | Cytotoxicity assay | Cytotoxicity against human MRC5 cells, CC50=49.33 μM | ||||
| human HUVEC cells | Function assay | 2 μM | 24 h | Inhibition of VEGFR2 glycosylation in human HUVEC cells at 2 uM after 24 hrs by Western blot analysis | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 13 mg/mL
(18.42 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 705.65031 | Formula | C35H38Cl2N8O4 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 84625-61-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | R 51211 | Smiles | CCC(C)N1C(=O)N(C=N1)C2=CC=C(C=C2)N3CCN(CC3)C4=CC=C(C=C4)OCC5COC(O5)(CN6C=NC=N6)C7=C(C=C(C=C7)Cl)Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
CYP3A4
(human liver microsomes) 6.1 nM
|
|---|---|
| In vitro |
Itraconazole is metabolized into hydroxy-itraconazole (OH-ITZ), a known in vivo metabolite of ITZ, and two new metabolites: keto-itraconazole (keto-ITZ) and N-desalkyl-itraconazole (ND-ITZ). This compound is a substrate for CYP3A in vitro and to characterize the metabolites generated. It exhibits an unbound Km of 3.9 nM for CYP3A. Its metabolites are as potent as or more potent CYP3A4 inhibitors than ITZ itself. This compound appears to act on the essential Hh pathway component Smoothened (SMO) by a mechanism distinct from that of cyclopamine and other known SMO antagonists, and prevents the ciliary accumulation of SMO normally caused by Hh stimulation. It is active against 60 clinical isolates of Aspergillus spp. with geometric mean (GM) MICs of 0.25 mg/mL. It acts primarily by impairing the synthesis of ergosterol, resulting in a defective fungal cell membrane with altered permeability and function. This chemical is effective for a wide variety of mycotic infections and some fungal meningeal infections. It has an affinity for mammalian cytochrome P-450 enzymes as well as for fungal P-450-dependent enzyme, and thus has the potential for clinically important interactions (e.g., astemizole, terfenadine, rifampin, oral contraceptives, H2 receptor antagonists, warfarin, cyclosporine).
|
| In vivo |
Itraconazole, like other Hh pathway antagonists, can suppress Hh pathway activity and the growth of medulloblastoma in a mouse allograft model.
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
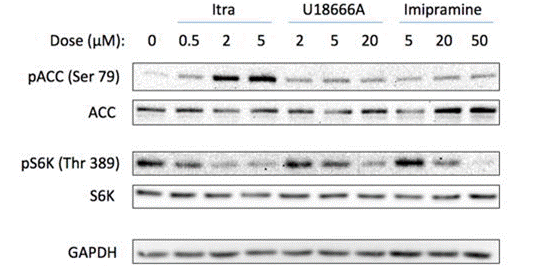
| Western blot | pACC / ACC / p-S6K / S6K GLI1 / GLI2 / p-AKT1 / AKT1 / Cyclin D1 |
|
28103683 |
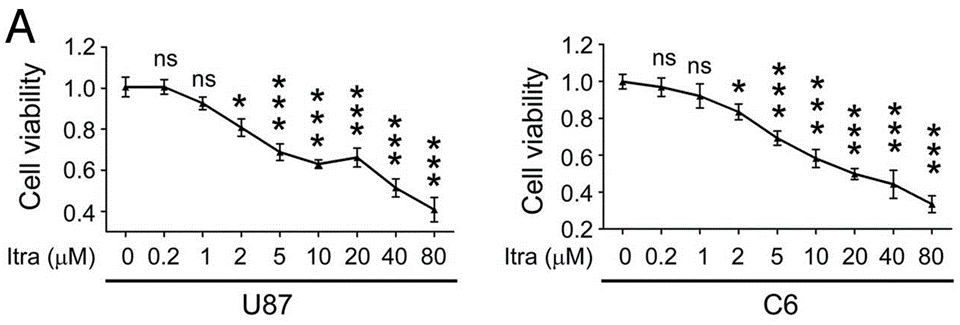
| Growth inhibition assay | Cell viability |
|
24905460 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05563766 | Not yet recruiting | Esophageal Adenocarcinoma|Esophageal Squamous Cell Carcinoma|Gastroesophageal Junction Carcinoma |
VA Office of Research and Development|Durham VA Health Care System|VA Palo Alto Health Care System|Portland VA Medical Center|VA Puget Sound Health Care System|Michael E. DeBakey VA Medical Center|VA Boston Healthcare System |
May 1 2024 | Phase 2 |
| NCT06357520 | Recruiting | Healthy Participants |
AstraZeneca |
April 16 2024 | Phase 1 |
| NCT06348888 | Recruiting | Advanced Non-small Cell Lung Cancer|EGFR Mutation|HER2 Mutation|Healthy Volunteers |
Bayer |
April 10 2024 | Phase 1 |
| NCT06362642 | Recruiting | Healthy Volunteers |
PMV Pharmaceuticals Inc |
March 28 2024 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
We are finding the best vehicle to administer it to mice. We want a mild vehicle (unlike DMSO) which resembles water, PBS, saline (for i.p/ injection) or methyl cellulose (for oral).
Answer:
We are not able to dissolve S2476 clearly without DMSO. For oral gavage, this compound can be dissolved in 1% CMC Na at 20mg/ml as a homogeneous suspension.






































