research use only
Enzastaurin PKC inhibitor
Cat.No.S1055
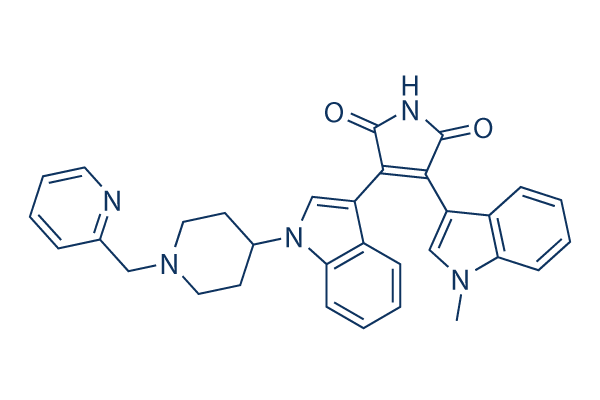
Chemical Structure
Molecular Weight: 515.61
Quality Control
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for MG 63 (6-TG R) cells | 29435139 | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 14 mg/mL
(27.15 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 515.61 | Formula | C32H29N5O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 170364-57-5 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | LY317615 | Smiles | CN1C=C(C2=CC=CC=C21)C3=C(C(=O)NC3=O)C4=CN(C5=CC=CC=C54)C6CCN(CC6)CC7=CC=CC=N7 | ||
Mechanism of Action
| Targets/IC50/Ki |
PKCβ
(Cell-free assay) 6 nM
PKCα
(Cell-free assay) 39 nM
PKCγ
(Cell-free assay) 83 nM
PKCε
(Cell-free assay) 110 nM
|
|---|---|
| In vitro |
Enzastaurin application results in a marked dose-dependent inhibition of growth in all MM cell lines investigated, including MM.1S, MM.1R, RPMI 8226 (RPMI), RPMI-Dox40 (Dox40), NCI-H929, KMS-11, OPM-2, and U266, with IC50 from 0.6-1.6 μM. This compound direct impacts human tumor cells, inducing apoptosis and suppressing proliferation in cultured tumor cells. It also suppresses the phosphorylation of GSK3βser9, ribosomal protein S6S240/244, and AKTThr308 while having no direct effect on VEGFR phosphorylation. This chemical increases apoptosis in malignant lymphocytes of CTCL. When combined with GSK3 inhibitors, it demonstrated an enhancement of cytotoxicity levels. Treatment with a combination of this compound and the GSK3 inhibitor AR-A014418 led to increased levels of β-catenin total protein and β-catenin-mediated transcription. Blocking of β-catenin-mediated transcription or small hairpin RNA (shRNA) knockdown of β-catenin induced the same cytotoxic effects as that of it plus AR-A014418. Additionally, treatment with it and AR-A014418 decreased the mRNA levels and surface expression of CD44. |
| Kinase Assay |
Kinase inhibition assays
|
|
The inhibition of PKCβII, PKCα, PKCε, or PKCγ activity by enzastaurin is determined using a filter plate assay format measuring 33P incorporation into myelin basic protein substrate. Reactions are done in 100 μL reaction volumes in 96-well polystyrene plates with final conditions as follows: 90 mM HEPES (pH 7.5), 0.001% Triton X-100, 4% DMSO, 5 mM MgCl2, 100 μM CaCl2, 0.1 mg/mL phosphatidylserine, 5 μg/mL diacetyl glyerol, 30 μM ATP, 0.005 μCi/μL 33ATP, 0.25 mg/mL myelin basic protein, serial dilutions of this compound (1-2,000 nM), and recombinant human PKCβII, PKCα, PKCε, or PKCγ enzymes (390, 169, 719, or 128 pM, respectively). Reactions are started by addition of the enzyme and incubated at room temperature for 60 minutes. They are then quenched with 10% H3PO4, transferred to multiscreen anionic phosphocellulose 96-well filter plates, incubated for 30 to 90 minutes, filtered and washed with 4 volumes of 0.5% H3PO4 on a vacuum manifold. Scintillation cocktail is added and plates are read on a Microbeta scintillation counter. IC50 values are determined by fitting a three-variable logistic equation to the 10-point dose-response data using ActivityBase 4.0.
|
|
| In vivo |
Treatment of xenografts with Enzastaurin and radiation produced greater reductions in density of microvessels than either treatment alone. The decrease in microvessel density corresponded to delayed tumor growth. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | p27 / Cyclin D1 / Bcl-2 / Survivin p-ERK / ERK / p-AKT / AKT PKCα / PKCβ / PKCδ / PKCε / PKCθ / p-PKCε / p-PKCθ p-eIF2a / eIF2a / p-ATF2 / ATF2 / CHOP / p21 / ATF6 / IRE1α |
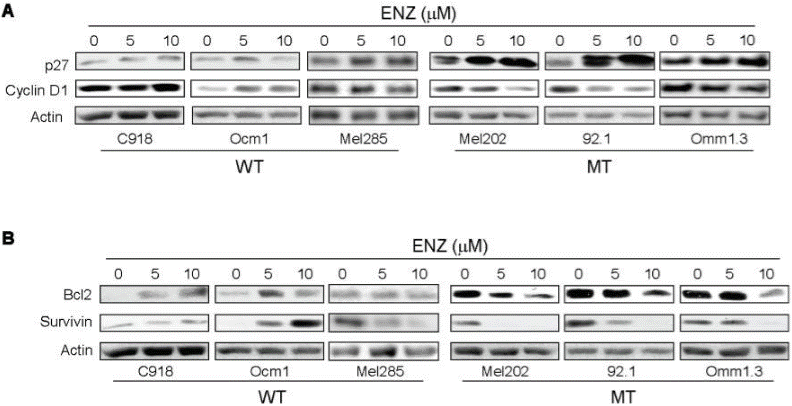
|
22253748 |
| Growth inhibition assay | Cell viability |
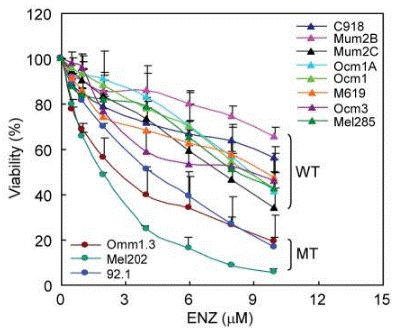
|
22253748 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03263026 | Completed | Diffuse Large B-Cell Lymphoma |
Denovo Biopharma LLC |
March 20 2018 | Phase 3 |
| NCT01432951 | Completed | Solid Tumor|Lymphoma Malignant |
Eli Lilly and Company |
November 2011 | Phase 1 |
| NCT01388335 | Completed | Solid Tumor|Lymphoma Malignant |
Eli Lilly and Company |
August 2011 | Phase 1 |
| NCT00744991 | Completed | Cutaneous T-Cell Lymphoma |
Eli Lilly and Company |
September 2008 | Phase 2 |
| NCT00709995 | Completed | Metastatic Renal Cell Carcinoma |
Eli Lilly and Company |
June 30 2008 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































