research use only
Bisindolylmaleimide I (GF109203X) PKC inhibitor
Cat.No.S7208
Chemical Structure
Molecular Weight: 412.48
Quality Control
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| RAW264.7 | Function assay | 24 hrs | Protection against Bacillus anthracis lethal toxin-mediated cytotoxicity in mouse RAW264.7 cells assessed as change in viability after 24 hrs by WST1 dye reduction assay, IC50=1.5μM | 17485504 | ||
| RAW264.7 | Function assay | Protection against Bacillus anthracis protective antigen and lethal toxin-diphtheria toxin chimeric protein mediated cytotoxicity in mouse RAW264.7 cells assessed as cell viability | 17485504 | |||
| CHO | Function assay | Inhibition of Bacillus anthracis anthrax protective antigen heptamer pre-pore to pore conversion in CMG2-expressing CHO cells | 19540764 | |||
| insect cells | Function assay | 120 mins | Inhibition of N-terminal GST tag LMTK3 kinase domain (133 to 415 amino acids) (unknown origin) expressed in insect cells incubated for 120 mins by radiometric fluorescent detection based CisBio KinEASE STK-S1 kit based assay, IC50=0.0001318μM | ChEMBL | ||
| Sf9 | Function assay | 30 min | Inhibition of GSK3beta (unknown origin) expressed in Sf9 cells using GS1 as substrate and [gamma32]ATP after 30 min by scinitllation counting, IC50=0.19055μM | ChEMBL | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 82 mg/mL
(198.79 mM)
Ethanol : 1 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 412.48 | Formula | C25H24N4O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 133052-90-1 | -- | Storage of Stock Solutions |
|
|
| Synonyms | GO 6850 | Smiles | CN(C)CCCN1C=C(C2=CC=CC=C21)C3=C(C(=O)NC3=O)C4=CNC5=CC=CC=C54 | ||
Mechanism of Action
| Features |
Greater selectivity than PKC inhibitor staurosporine. GF109203X is a chemical probe for studying PKC signal transduction pathways. Potential for use in a variety of cancers.
|
|---|---|
| Targets/IC50/Ki |
PKCβ2
(Cell-free assay) 16 nM
PKCβ1
(Cell-free assay) 17 nM
PKCα
(Cell-free assay) 20 nM
PKCγ
(Cell-free assay) 20 nM
|
| In vitro |
Bisindolylmaleimide I (GF109203X) is an ATP-competitive PKC inhibitor that prevents platelet aggregation induced by stimuli activating PKC, and has potential as a tool for studying PKC involvement in signal transduction pathways. It also produces reversal activity on P-glycoprotein and MRP-mediated multidrug resistance. PKC inhibition by this compound significantly reduces carbachol-stimulated ERK1/2 activation and the subsequent proliferation of SNU-407 colon cancer cells.
|
| Kinase Assay |
Assay of protein kinase C
|
|
Bisindolylmaleimide I (GF109203X) is arrayed by measuring 32PI transferred from [gamma-32PI] ATP to lysine-rich histone type Ill-s. The reaction mixture (80 μL) contained 50 mM Tris-HCI. pH 7.4, 100 μM CaCl2, 10 mM MgCI2, 37.5 μL/mL histone type Ill-s, 10 μM [gamma-32PI] ATP (1250 cpm/pmol), 31 μM bovine brain phosphatidylserine and 0.5 μM 1,2 sn-dioleylglycerol. Fifteen μL of purified PKC (final concentration in assay 0.38 μg/mL) is added to the incubation mixture. After 10 min at 30°C, the reaction is stopped by addition of 30 μL of casein 30 mg/mL and 0.9 ml of 12% trichloroacetic acid. The acid precipitable material is collected by centrifugation, dissolved in 1N NaOH (100μL) and precipitated again with 1 ml of 12% trichloroacetic acid. The pellet is dissolved in 1N NaOH (100μL) and 32P incorporation is measured by scintillation counting in Aquasol.
|
|
| In vivo |
Bisindolylmaleimide I (GF109203X) dose-dependently inhibits BK-induced mechanical allodynia in Wistar rats at a dose of 10 μg/mouse (i.pl.).
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | pMAPK / pCREB / pAKT p-HDAC5 / HDAC5 p-PKD / PKD |
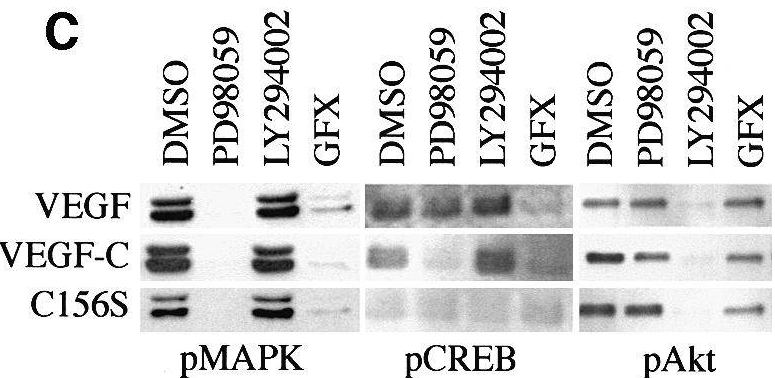
|
11532940 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































