|
How to Cite 1. For In-Text Citation (Materials & Methods): 2. For Key Resources Table: |
||
|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. We strive to reply to |
Technical Data
| Formula | C32H29N5O2 |
||||||||||
| Molecular Weight | 515.61 | CAS No. | 170364-57-5 | ||||||||
| Solubility (25°C)* | In vitro | DMSO | 14 mg/mL (27.15 mM) | ||||||||
| Water | Insoluble | ||||||||||
| Ethanol | Insoluble | ||||||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||||||
Preparing Stock Solutions
Biological Activity
| Description | Enzastaurin is a potent PKCβ selective inhibitor with IC50 of 6 nM in cell-free assays, 6- to 20-fold selectivity against PKCα, PKCγ and PKCε. Phase 3. | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Targets |
|
||||||||
| In vitro | Enzastaurin application results in a marked dose-dependent inhibition of growth in all MM cell lines investigated, including MM.1S, MM.1R, RPMI 8226 (RPMI), RPMI-Dox40 (Dox40), NCI-H929, KMS-11, OPM-2, and U266, with IC50 from 0.6-1.6 μM. This compound direct impacts human tumor cells, inducing apoptosis and suppressing proliferation in cultured tumor cells. It also suppresses the phosphorylation of GSK3βser9, ribosomal protein S6S240/244, and AKTThr308 while having no direct effect on VEGFR phosphorylation. This chemical increases apoptosis in malignant lymphocytes of CTCL. When combined with GSK3 inhibitors, it demonstrated an enhancement of cytotoxicity levels. Treatment with a combination of this compound and the GSK3 inhibitor AR-A014418 led to increased levels of β-catenin total protein and β-catenin-mediated transcription. Blocking of β-catenin-mediated transcription or small hairpin RNA (shRNA) knockdown of β-catenin induced the same cytotoxic effects as that of it plus AR-A014418. Additionally, treatment with it and AR-A014418 decreased the mRNA levels and surface expression of CD44. |
||||||||
| In vivo | Treatment of xenografts with Enzastaurin and radiation produced greater reductions in density of microvessels than either treatment alone. The decrease in microvessel density corresponded to delayed tumor growth. |
Protocol (from reference)
| Kinase Assay: |
|
|---|---|
| Cell Assay: |
|
| Animal Study: |
|
References
|
Customer Product Validation
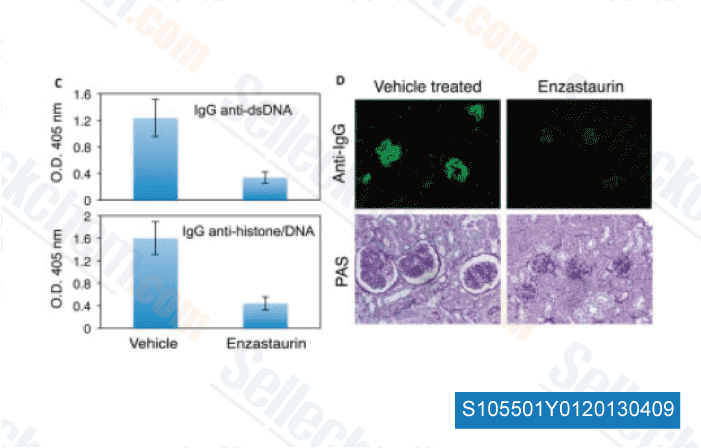
-
Data from [ Arthritis Rheum , 2013 , 65, 1022-31 ]
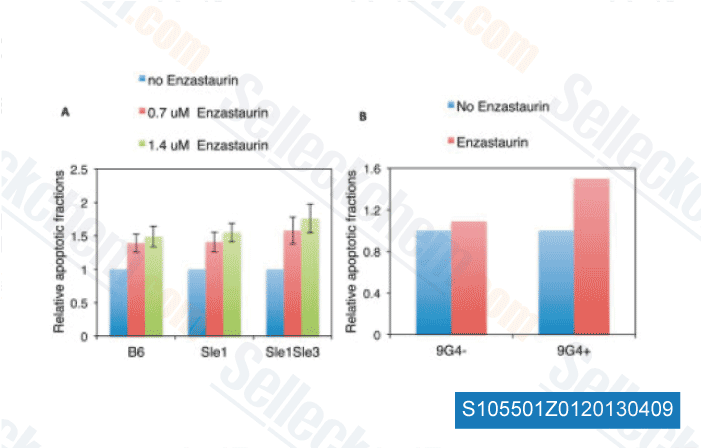
-
Data from [ Arthritis Rheum , 2013 , 65, 1022-31 ]
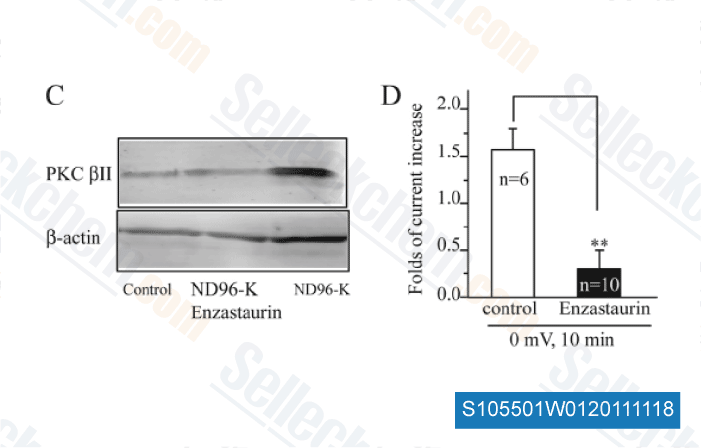
-
Data from [ J Biol Chem , 2011 , 286, 39760-7 ]
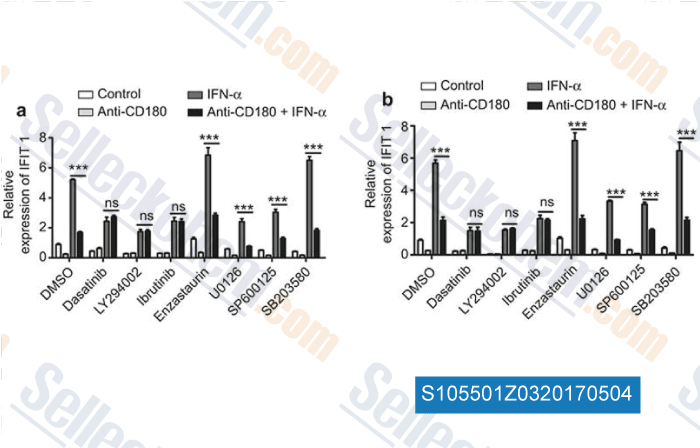
-
Data from [ , , Cell Mol Immunol, 2017, 14(2):192-202 ]
Selleck's Enzastaurin Has Been Cited by 67 Publications
| IDH status dictates oHSV mediated metabolic reprogramming affecting anti-tumor immunity [ Nat Commun, 2025, 16(1):3874] | PubMed: 40274791 |
| Glioblastoma Tumor Microtubes and Brain Fatty Acid-binding Protein: Path to Directional Infiltration [ Neuro Oncol, 2025, noaf200] | PubMed: 40891350 |
| A patient-derived T cell lymphoma biorepository uncovers pathogenetic mechanisms and host-related therapeutic vulnerabilities [ Cell Rep Med, 2025, S2666-3791(25)00102-8] | PubMed: 40147445 |
| A genome-wide association study identified PRKCB as a causal gene and therapeutic target for Mycobacterium avium complex disease [ Cell Rep Med, 2025, S2666-3791(24)00694-3] | PubMed: 39848245 |
| Single-cell analysis reveals transcriptomic features and therapeutic targets in primary pulmonary lymphoepithelioma-like carcinoma [ Commun Biol, 2025, 8(1):394] | PubMed: 40057671 |
| Patient-derived rhabdomyosarcoma cells recapitulate the genetic and transcriptomic landscapes of primary tumors [ iScience, 2024, 27(10):110862] | PubMed: 39319271 |
| Epoxytiglianes induce keratinocyte wound healing responses via classical protein kinase C activation to promote skin re-epithelialization [ Biochem Pharmacol, 2024, 230(Pt 2):116607] | PubMed: 39489221 |
| PKCβII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis [ Nat Cell Biol, 2022, 24(1):88-98] | PubMed: 35027735 |
| Targeting a splicing-mediated drug resistance mechanism in prostate cancer by inhibiting transcriptional regulation by PKCβ1 [ Oncogene, 2022, 10.1038/s41388-022-02179-z] | PubMed: 35087237 |
| Inhibition of IκB Kinase Is a Potential Therapeutic Strategy to Circumvent Resistance to Epidermal Growth Factor Receptor Inhibition in Triple-Negative Breast Cancer Cells [ Cancers (Basel), 2022, 14(21)5215] | PubMed: 36358633 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
