IFN inhibitors, a class of biological or small-molecule agents designed to modulate the interferon (IFN) signaling pathway, have emerged as pivotal tools in both basic immunology research and clinical translational studies. Interferons, a family of pleiotropic cytokines, play essential roles in regulating innate and adaptive immune responses, antiviral defense, and immune surveillance against malignancies. However, dysregulated or excessive IFN signaling is closely associated with the pathogenesis of numerous autoimmune and inflammatory diseases, such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and type 1 diabetes. In this context, IFN inhibitors have garnered significant attention for their potential to attenuate aberrant IFN activity, thereby restoring immune homeostasis.
IFN inhibitors/activators
Other Immunology & Inflammation Related Inhibitors
| Cat.No. | Product Name | Information | Product Use Citations | Product Validations |
|---|---|---|---|---|
| S8879 | BMS-986165 (Deucravacitinib) | Deucravacitinib (BMS-986165) is a highly potent and selective allosteric inhibitor of Tyk2 with a Ki value of 0.02 nM for binding to the Tyk2 pseudokinase domain. It is highly selective against a panel of 265 kinases and pseudokinases. |
|
|
| S8133 | Resiquimod (R-848) | Resiquimod (R-848, S28463) is an immune response modifier that acts as a potent TLR 7/TLR 8 agonist, inducing the upregulation of cytokines such as TNF-α, IL-6 and IFN-α. This compound reduces hepatitis C virus (HCV) infection. Phase 2. |
|
|
| S1537 | Vadimezan (DMXAA) | Vadimezan (DMXAA) is a vascular disrupting agents (VDA) and competitive inhibitor of DT-diaphorase with Ki of 20 μM and IC50 of 62.5 μM in cell-free assays, respectively. It is also a STING agonist with potential antineoplastic activity, potently inducing IFN-β but relatively low TNF-α expression in vitro. This compound has antiviral activity. Phase 3. |
|

|
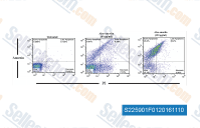
| S2259 | Aloe-emodin | Aloe-emodin (NSC 38628, Rhabarberone) is an interferon-inducing agent with IC50 of about 1 μg/mL for JEV and of about 0.33 μg/mL for EV71. |
|
|
| S0289 | KIN1148 | KIN1148 is an agonist of interferon regulatory factor 3 (IRF3) that induces dose-dependent IRF3 nuclear translocation and specific activation of IRF3-responsive promoters. This compound is an influenza vaccine adjuvant that enhances flu vaccine efficacy. |
|
|
| E5781 | 2',3'-cGAMP | 2',3'-cGAMP (2'-3'-cyclic GMP-AMP) is an endogenous cyclic GMP-AMP produced in mammalian cells in response to cytoplasmic DNA. It binds STING with high affinity Kd of 3.79 nM and is a potent inducer of interferon-β (IFNβ). |
|
|
| E2982 | IFN alpha-IFNAR-IN-1 hydrochloride | IFN alpha-IFNAR-IN-1 hydrochloride is a nonpeptidic, low-molecular-weight inhibitor that inhibits the interaction between IFN-α and IFNAR. This compound inhibits modified Vaccinia virus ankara (MVA)-induced IFN-α responses in murine bone-marrow-derived, Flt3- L-differentiated pDC cultures (BM-pDCs) with IC50 value of 2-8 μM. | ||
| S3827 | Royal jelly acid | Royal jelly acid (10-Hydroxy-trans-2-decenoic acid, 10H2DA) is the principal lipid component in royal jelly which is the food for queen and larvae honeybees. It is involved in several treatment processes of autoimmune and inflammatory diseases, including inhibition of lipopolysaccharide (LPS)- and interferon (IFN)-γ-stimulated macrophage responses, inhibition of T-cell proliferation and anti-rheumatoid activity. | ||
| S5020 | Tilorone dihydrochloride | Tilorone dihydrochloride is a broad-spectrum, orally active antiviral agent that activates interferon production. It has antitumor, anti-inflammatory properties. | ||
| E0448 | RO8191 | RO8191 (CDM-3008, RO4948191), an imidazonaphthyridine compound, is an orally active and potent agonist of interferon (IFN) receptor. This compound binds directly to the IFNα/β receptor 2 (IFNAR2), activating IFN-stimulated genes (ISGs) expression and the phosphorylation of JAK/STAT signaling. | ||
Interferons: Classification and Functional Basis for IFN Inhibitor Development
To comprehensively understand the mechanism of action of IFN inhibitors, it is fundamental to clarify the classification and functional characteristics of interferons. Interferons are categorized into three major types: Type I (including IFN-alpha, IFN-beta, IFN-epsilon, etc.), Type II (solely IFN-gamma), and Type III (IFN-lambda 1–3). Among these, IFN-alpha and IFN-gamma are the most extensively studied subtypes in the context of IFN inhibitor research, owing to their prominent roles in immune dysregulation and disease progression.
IFN-alpha and IFN-gamma: Key Subtypes in Immune Pathophysiology
IFN-alpha, a prototype of Type I IFNs, is primarily produced by plasmacytoid dendritic cells (pDCs) in response to viral nucleic acids or microbial pathogens. It exerts antiviral effects by inducing the expression of hundreds of interferon-stimulated genes (ISGs), which inhibit viral replication and assembly. Additionally, IFN-alpha modulates the activation and differentiation of T cells, B cells, and macrophages, thereby shaping adaptive immune responses. However, chronic overproduction of IFN-alpha is a hallmark of SLE, where it drives the production of autoantibodies and promotes tissue inflammation. On the other hand, IFN-gamma, the only Type II IFN, is mainly secreted by activated T helper 1 (Th1) cells, cytotoxic T lymphocytes (CTLs), and natural killer (NK) cells. It plays a critical role in enhancing macrophage phagocytosis, upregulating major histocompatibility complex (MHC) molecules, and promoting Th1 polarization. Dysregulated IFN-gamma signaling is implicated in autoimmune diseases such as multiple sclerosis (MS) and Crohn's disease, as well as chronic inflammatory conditions. The distinct yet overlapping functional roles of IFN-alpha and IFN-gamma provide precise targets for the design of IFN inhibitors with subtype-specific or pan-inhibitory activities.
Functional Implications of IFN Signaling in Disease: Rationale for Inhibition
The functional diversity of interferons dictates their dual role in health and disease. Under physiological conditions, IFN signaling is tightly regulated to balance antiviral defense and immune tolerance. However, in pathological states, sustained activation of the IFN pathway disrupts this balance, leading to immune-mediated tissue damage. For instance, in SLE patients, the "IFN signature"—a distinct gene expression profile characterized by upregulated ISGs—correlates with disease severity and organ involvement. Similarly, in MS, IFN-gamma promotes the infiltration of inflammatory cells into the central nervous system (CNS), exacerbating demyelination. These observations underscore the therapeutic potential of IFN inhibitors, which aim to abrogate the pathogenic effects of excessive IFN signaling while preserving beneficial immune functions. Basic research on the functional consequences of IFN signaling has thus laid the groundwork for the development of targeted IFN inhibitors.
Mechanism of Action of IFN Inhibitors: Targeting Key Nodes in the IFN Signaling Pathway
The mechanism of action of IFN inhibitors is centered on disrupting critical steps in the IFN signaling cascade, which commences with the binding of IFNs to their cognate receptors on target cells. Type I IFNs (including IFN-alpha) bind to the IFN-alpha/beta receptor (IFNAR), composed of IFNAR1 and IFNAR2 subunits, while IFN-gamma binds to the IFN-gamma receptor (IFNGR), consisting of IFNGR1 and IFNGR2. Receptor activation triggers a downstream signaling cascade involving Janus kinases (JAKs) and signal transducers and activators of transcription (STATs), ultimately leading to the transcription of ISGs. IFN inhibitors target various nodes in this pathway, including IFN ligands, receptors, JAKs, STATs, and downstream signaling molecules.
Ligand-Targeted Inhibitors: Neutralizing IFN-alpha and IFN-gamma
One of the most straightforward strategies for inhibiting IFN activity is the use of neutralizing antibodies that bind directly to IFN ligands (IFN-alpha or IFN-gamma), preventing their interaction with receptors. For example, anti-IFN-alpha monoclonal antibodies (mAbs) such as anifrolumab and sifalimumab have been extensively studied in clinical trials for SLE. These antibodies recognize multiple subtypes of IFN-alpha, thereby blocking their ability to activate the IFNAR pathway. Preclinical studies have demonstrated that anti-IFN-alpha mAbs can reduce the expression of ISGs and attenuate autoimmune phenotypes in murine models of SLE. Similarly, anti-IFN-gamma mAbs, such as emapalumab, have shown efficacy in the treatment of hemophagocytic lymphohistiocytosis (HLH), a life-threatening hyperinflammatory syndrome driven by excessive IFN-gamma production. The mechanism of action of these ligand-targeted inhibitors is highly specific, as they directly neutralize the pathogenic IFN subtypes without affecting other components of the immune system.
Receptor and Intracellular Signaling-Targeted Inhibitors
Another major class of IFN inhibitors targets the IFN receptors or the intracellular JAK-STAT signaling pathway. Receptor antagonists, such as soluble IFNAR or IFNGR fragments, compete with native receptors for IFN binding, thereby inhibiting receptor activation. Although these agents have shown promise in preclinical studies, their clinical application is limited by issues such as low stability and immunogenicity. In contrast, JAK inhibitors (JAKinibs), which target the JAK enzymes involved in IFN signaling (JAK1, JAK2, TYK2), have emerged as a versatile class of IFN inhibitors. For example, tofacitinib (a pan-JAK inhibitor) and baricitinib (a JAK1/JAK2 inhibitor) have been approved for the treatment of RA and SLE, respectively. These inhibitors block the phosphorylation and activation of STATs, thereby suppressing the transcription of ISGs induced by both IFN-alpha and IFN-gamma. The mechanism of action of JAKinibs is broader than ligand-targeted inhibitors, as they can modulate multiple cytokine pathways beyond IFNs, which may contribute to their therapeutic efficacy but also increase the risk of off-target effects. Recent research has focused on developing selective JAK inhibitors (e.g., TYK2 inhibitors) to improve specificity and reduce adverse reactions.
IFN Inhibitors in Medication Development and Therapy: Translational Research Advances
The translational potential of IFN inhibitors has been extensively explored in the development of medications for autoimmune, inflammatory, and infectious diseases. The transition from basic research on the mechanism of action of IFN inhibitors to clinical therapy involves rigorous preclinical validation, phase I–III clinical trials, and post-marketing surveillance. This section highlights the key advances in medication development and therapeutic application of IFN inhibitors, with a focus on IFN-alpha and IFN-gamma targeted agents.
Medication Development of IFN Inhibitors: From Preclinical to Clinical Stages
Preclinical research on IFN inhibitors typically involves in vitro studies using cell lines (e.g., pDCs, T cells, or patient-derived cells) and in vivo studies using animal models of disease. These studies evaluate the efficacy, safety, and pharmacokinetic properties of potential inhibitors, providing critical data for advancing candidates to clinical trials. For example, anifrolumab, a fully human anti-IFN-alpha mAb, underwent extensive preclinical testing in murine models of SLE, where it reduced autoantibody levels, glomerulonephritis, and skin lesions. Subsequent phase III clinical trials (e.g., TULIP 1 and TULIP 2) demonstrated that anifrolumab significantly improved disease activity in SLE patients with moderate to severe disease, leading to its approval by the U.S. Food and Drug Administration (FDA) in 2021. Similarly, emapalumab, an anti-IFN-gamma mAb, was validated in preclinical models of HLH and subsequently approved for the treatment of pediatric and adult HLH patients who are refractory to conventional therapy. These examples illustrate the successful translation of basic research on IFN inhibitors into clinically approved medications.
Therapeutic Efficacy and Challenges of IFN Inhibitors in Clinical Practice
The therapeutic application of IFN inhibitors has shown promising results in several autoimmune and inflammatory diseases. In addition to SLE and HLH, IFN inhibitors are being investigated in clinical trials for MS, RA, Sjögren's syndrome, and systemic sclerosis. For example, in MS, IFN-gamma inhibitors are being tested to reduce neuroinflammation and demyelination, as IFN-gamma is thought to contribute to the pathogenesis of the disease. However, the clinical use of IFN inhibitors also faces several challenges. First, the heterogeneity of disease phenotypes and the variable role of IFN signaling in different patients can lead to variable therapeutic responses. For instance, not all SLE patients exhibit the IFN signature, and thus may not benefit from IFN-alpha inhibitors. Second, long-term use of IFN inhibitors may be associated with adverse effects, such as increased risk of infections (due to suppression of antiviral immunity), hematological abnormalities, and gastrointestinal disorders. Third, the high cost of biological IFN inhibitors (e.g., monoclonal antibodies) limits their accessibility. Ongoing research aims to address these challenges by developing personalized therapeutic strategies (e.g., selecting patients based on IFN signature status), improving the specificity of inhibitors, and exploring novel formulations to reduce costs.
Future Directions in IFN Inhibitor Research
Despite significant advances in the field, several areas of IFN inhibitor research remain to be explored. Future studies will focus on elucidating the precise role of different IFN subtypes and ISGs in disease pathogenesis, which will enable the development of more selective and effective inhibitors. Additionally, the combination of IFN inhibitors with other immunomodulatory agents (e.g., checkpoint inhibitors, corticosteroids) is a promising strategy to enhance therapeutic efficacy in complex diseases. Furthermore, the application of IFN inhibitors in infectious diseases, such as COVID-19 (where excessive IFN signaling contributes to severe inflammation), is an emerging area of research. Finally, the development of small-molecule IFN inhibitors, which offer advantages such as oral administration and lower production costs compared to biological agents, will expand the clinical utility of IFN-targeted therapies.
In conclusion, IFN inhibitors represent a valuable class of therapeutic agents with significant potential in the treatment of immune-mediated diseases. Basic research on the function and mechanism of action of interferons (particularly IFN-alpha and IFN-gamma) has provided the foundation for the development of targeted IFN inhibitors. Translational studies have successfully converted preclinical findings into clinically approved medications, and ongoing research continues to address the challenges associated with their use. As our understanding of the IFN signaling pathway and disease pathogenesis deepens, IFN inhibitors will undoubtedly play an increasingly important role in personalized medicine and immunotherapy.





































