research use only
TAK-901 Aurora Kinase inhibitor
Cat.No.S2718
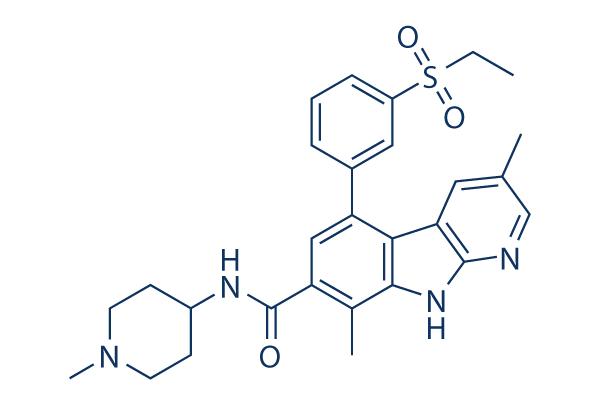
Chemical Structure
Molecular Weight: 504.64
Quality Control
| Related Targets | CDK HSP PD-1/PD-L1 ROCK Wee1 DNA/RNA Synthesis Microtubule Associated Ras KRas Casein Kinase |
|---|---|
| Other Aurora Kinase Inhibitors | Hesperadin Barasertib-HQPA (AZD2811) Alisertib (MLN8237) Tozasertib (VX-680) ZM 447439 MLN8054 Danusertib (PHA-739358) MK-5108 TCS7010 (Aurora A Inhibitor I) AMG-900 |
Solubility
|
In vitro |
DMSO
: 101 mg/mL
(200.14 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 504.64 | Formula | C28H32N4O3S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 934541-31-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CCS(=O)(=O)C1=CC=CC(=C1)C2=CC(=C(C3=C2C4=C(N3)N=CC(=C4)C)C)C(=O)NC5CCN(CC5)C | ||
Mechanism of Action
| Targets/IC50/Ki |
JAK3
1.2 nM
c-Src
1.3 nM
CLK2
<2.0 nM
FGR
<2.0 nM
YES1
<2.0 nM
LRRK2
3.0 nM
FLT3
5.0 nM
Fyn
5.3 nM
ARG
5.6 nM
Axl
5.8 nM
Hck
5.8 nM
SNF1LK2
6.0 nM
Abl
6.4 nM
RET
6.5 nM
TrkA
6.7 nM
LCK
6.8 nM
PTK5
7.1 nM
Fms
7.2 nM
FGFR2
7.3 nM
EphB1
8.9 nM
Abl (T315I)
9.0 nM
EphA1
9.1 nM
ARK5
9.1 nM
ITK
12 nM
ALK2
13 nM
CDK7
14 nM
BLK
14 nM
Aurora B-INCENP
15 nM
JAK2
17 nM
EphB2
18 nM
Aurora A-TPX2
21 nM
STK16
24 nM
EphA2
24 nM
BRK
25 nM
EphB4
27 nM
TNK2
27 nM
FGFR1
28 nM
EphA4
31 nM
STK33
35 nM
CLK1
45 nM
AMPK
46 nM
FES
48 nM
SLK
49 nM
Chk2
50 nM
TYK2
54 nM
BTK
55 nM
FAK2
55 nM
c-Kit
58 nM
VEGFR2
60 nM
FGFR3
65 nM
ALK5
78 nM
MAP3K9
80 nM
CLK3
88 nM
JAK1
93 nM
|
|---|---|
| In vitro |
TAK-901 inhibits Aurora A-TPX2 and Aurora B-INCENP in tight binding, time-dependent manner. Dissociation of this compound from Aurora B-INCENP is slow with at 1/2 of 920 minutes, and the affinity constant for its binding to Aurora B-INCENP is determined to be 0.02 nM. It induces inhibition of cell proliferation in cultured human cancer cell lines from different tissues with IC50s ranging from 40 to 500nM. Consistent with Aurora B inhibition, this chemical treatment produces polyploidy in human PC3 prostate cancer and HL60 acute myeloid leukemia cells as measured by immunofluorescence and flow cytometry. Examination of a broad panel of kinases reveals that multiple kinases, including FLT3, FGFR and the Src family kinases, are inhibited by this agent with IC50 values similar to those for Aurora A and B. In cells, it suppresses the Flt3 and FGFR2 autophosphorylation with IC50 values close to that of Aurora B as measured by cellular histone H3 phosphorylation, whereas the IC50s for inhibition of cellular Src and Bcr Abl are 20-fold weaker. In a panel of pathway specific reporter-based cell models, it inhibits the NFkB and JAK/STAT pathways with submicromolar potency.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT00935844 | Completed | Advanced Solid Tumors|Lymphoma |
Millennium Pharmaceuticals Inc. |
October 2009 | Phase 1 |
| NCT00807677 | Completed | Acute Myeloid Leukemia|Acute Lymphoblastic Leukemia|Chronic Myelogenous Leukemia|Chronic Lymphocytic Leukemia|Multiple Myeloma|Waldenstrom''s Macroglobulinemia|Myelodysplastic Syndrome|Philadelphia Chromosome-negative CML|Myeloid Metaplasia|Myelofibrosis|Advanced Polycythemia|Non-Hodgkins Lymphoma |
Millennium Pharmaceuticals Inc. |
March 2009 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































