- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Palonosetron 5-HT Receptor antagonist
Cat.No.S5740
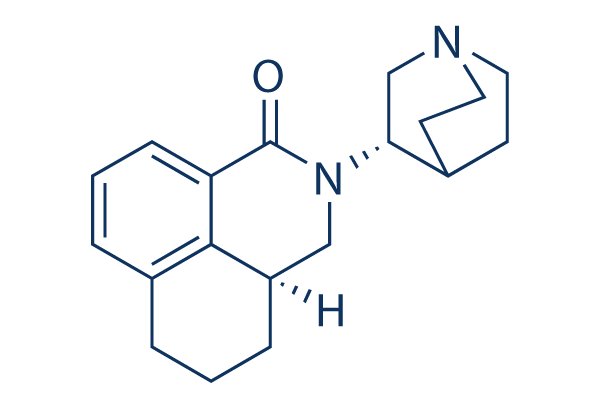
Chemical Structure
Molecular Weight: 296.41
Jump to
Quality Control
Solubility
|
In vitro |
DMSO
: 59 mg/mL
(199.04 mM)
|
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 296.41 | Formula | C19H24N2O |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 135729-61-2 | -- | Storage of Stock Solutions |
|
|
| Synonyms | RS25259, RS 25259 197 | Smiles | C1CC2CN(C(=O)C3=CC=CC(=C23)C1)C4CN5CCC4CC5 | ||
Mechanism of Action
| Targets/IC50/Ki |
5-HT3 receptor
0.17 nM(Ki)
|
|---|---|
| In vitro |
This compound is a 5-HT3 receptor antagonist with a high binding affinity for this receptor and little or no affinity for other receptors. |
| In vivo |
Palonosetron has a longer half-life and a higher binding affinity than the first-generation 5-HT3 receptor antagonists. After intravenous dosing of this compound in healthy subjects and cancer patients, an initial decline in plasma concentration is followed by a slow elimination from the body. Mean maximum plasma concentration and area under the concentration-time curves are generally dose-proportional over the dose range of 0.3 to 90 μg/kg in healthy subjects and in cancer patients. This compound has a volume of distribution of approximately 8.3 ± 2.5 L/kg and is 62% bound to plasma proteins. It is eliminated from the body through renal excretion and metabolic pathways. The mean terminal elimination half-life is approximately 40 hours. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04486157 | Completed | Healthy |
HK inno.N Corporation |
March 18 2021 | Phase 1 |
| NCT04466046 | Completed | Postoperative Nausea and Vomiting |
Daegu Catholic University Medical Center |
September 4 2019 | -- |
| NCT03148704 | Unknown status | Palonosetron |
Cttq |
March 8 2017 | -- |
| NCT00982995 | Terminated | Nausea|Vomiting|Terminally Ill |
University of Michigan Rogel Cancer Center |
November 2010 | Phase 2 |
| NCT01074697 | Completed | Nausea|Vomiting|Genital Neoplasms Female |
Odense University Hospital|Helsinn Healthcare SA |
April 2010 | Phase 3 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































