research use only
Nuciferine 5-HT Receptor antagonist
Cat.No.S3821
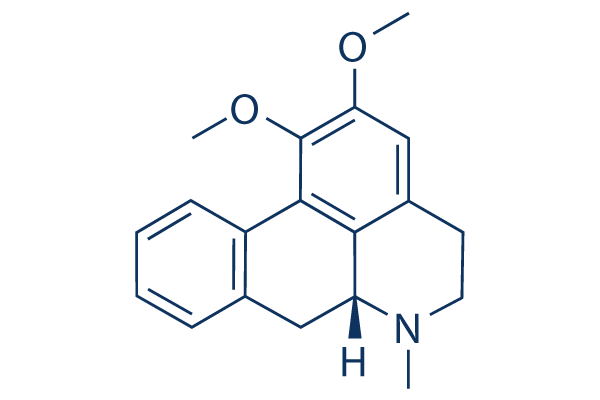
Chemical Structure
Molecular Weight: 295.38
Quality Control
| Related Targets | Adrenergic Receptor AChR COX Calcium Channel Histamine Receptor Dopamine Receptor GABA Receptor TRP Channel Cholinesterase (ChE) GluR |
|---|---|
| Other 5-HT Receptor Inhibitors | WAY-100635 Maleate Puerarin Serotonin (5-HT) HCl SB269970 HCl Ketanserin BRL-15572 Dihydrochloride RS-127445 Flopropione Azacyclonol BRL-54443 |
Solubility
|
In vitro |
DMSO
: 7 mg/mL
(23.69 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 295.38 | Formula | C19H21NO2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 475-83-2 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Sanjoinine E, (-)-Nuciferine, VLT 049 | Smiles | CN1CCC2=CC(=C(C3=C2C1CC4=CC=CC=C43)OC)OC | ||
Mechanism of Action
| In vitro |
Nuciferine inhibits the growth of MDA-MB-231 and MCF-7 human breast cancer cells by inducing apoptosis and inhibiting proliferation via cell cycle arrest. This compound inhibits the receptor activator of nuclear factor kappa-B ligand- (RANKL-) induced osteoclast differentiation in mouse bone marrow macrophage cells and mature osteoclast-mediated bone resorption. It reduces the viability of SY5Y human neuroblastoma cells and CT26 murine colon cancer cells, and inhibits nicotine-induced non-small-cell lung cancer progression.
|
|---|---|
| In vivo |
Nuciferine is found to decrease serum urate levels and improve kidney function, as well as inhibit system and renal interleukin-1β (IL-1β) secretion in potassium oxonate-induced hyperuricemic mice. Furthermore, this compound reverses expression alteration of renal urate transporter 1 (URAT1), glucose transporter 9 (GLUT9), ATP-binding cassette, subfamily G, membrane 2 (ABCG2), organic anion transporter 1 (OAT1), organic cation transporter 1 (OCT1), and organic cation/carnitine transporters 1/2 (OCTN1/2) in hyperuricemic mice. It also suppresses renal activation of TLR4/MyD88/NF-κB signaling and NOD-like receptor family, NLRP3 inflammasome to reduce serum and renal IL-1β levels in hyperuricemic mice with renal inflammation reduction.
|
References |
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































