research use only
LDN-193189 Dihydrochloride BMP Signaling inhibitor
Cat.No.S7507
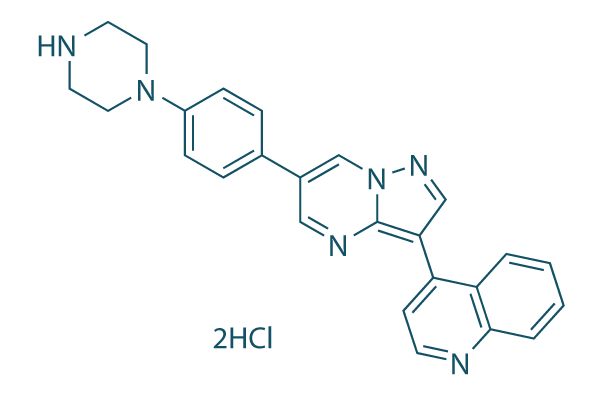
Chemical Structure
Molecular Weight: 479.4
Quality Control
| Related Targets | PKC ROCK Bcr-Abl |
|---|---|
| Other TGF-beta/Smad Inhibitors | SB431542 LDN-193189 Galunisertib (LY2157299) LY2109761 RepSox (E-616452) A-83-01 Vactosertib (TEW-7197) SRI-011381 (C381) SB-525334 SIS3 Hydrochloride |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| EOC216 | Cell viability assay | 0.1, 1, 2, 5, 10 μM | 1, 3, 5, 7, 9 days | exhibited a dose dependent LDN-induced decrease in viability | 25227893 | |
| PC3 | Function assay | 500 nM | LDN-193189 repressed activation of Smad1/5/8, and also repressed P-Smad3 levels | 22452883 | ||
| C2C12 | Function assay | 0.5 μM | 1 day | LDN-193189 promotes myogenesis | 25368322 | |
| C2C12 | Function assay | 30 mins | Inhibition of BMP6-induced ALK2 transcriptional activity in mouse C2C12 cells expressing BRE-Luc after 30 mins by luciferase reporter gene assay, EC50 = 0.014 μM. | 30227946 | ||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| fibroblast cells | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for control Hh wild type fibroblast cells | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh30 cells | 29435139 | |||
| C2C12 | Function assay | 0.1 uM | Inhibition of ALK5 in mouse C2C12 cells assessed as decrease in TGFbeta1-induced Smad1/5 phosphorylation at 0.1 uM by Western blot method | 28103025 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
Water : 24 mg/mL
DMSO
: 12 mg/mL
(25.03 mM)
Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 479.4 | Formula | C25H24Cl2N6 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1435934-00-1 | -- | Storage of Stock Solutions |
|
|
| Synonyms | DM-3189 2HCl | Smiles | C1CN(CCN1)C2=CC=C(C=C2)C3=CN4C(=C(C=N4)C5=CC=NC6=CC=CC=C56)N=C3.Cl.Cl | ||
Mechanism of Action
| Features |
Selective BMP type I receptor inhibitor.
|
|---|---|
| Targets/IC50/Ki |
ALK1
(Cell-free assay) 0.8 nM
ALK2
(Cell-free assay) 0.8 nM
ALK3
(Cell-free assay) 5.3 nM
ALK6
(Cell-free assay) 16.7 nM
|
| In vitro |
LDN193189 potently inhibits BMP4-mediated Smad1, Smad5 and Smad8 activation with IC50 of 5 nM, and efficiently inhibits transcriptional activity of the BMP type I receptors ALK2 and ALK3 with IC50 of 5 nM and 30 nM, respectively. Furthermore, LDN193189 also shows the inhibitory effect on the transcriptional activity induced by either constitutively active ALK2R206H or ALK2Q207D mutant proteins. A recent study shows that LDN-193189 blocks the production of reactive oxygen species induced by oxidized LDL during atherogenesis in human aortic endothelial cells. |
| Kinase Assay |
Alkaline phosphatase activity
|
|
C2C12 cells are seeded into 96-well plates at 2,000 cells per well in DMEM supplemented with 2% FBS. The wells are treated in quadruplicate with BMP ligands and LDN-193189 or vehicle. The cells are collected after 6 days in culture in 50 μL Tris-buffered saline and 1% Triton X-100. The lysates are added to p-nitro-phenylphosphate reagent in 96-well plates for 1 hours and then evaluated alkaline phosphatase activity (absorbance at 405 nm). Cell viability and quantity are measured by Cell Titer Aqueous One (absorbance at 490 nm), using replicate wells treated identically to those used for alkaline phosphatase measurements.
|
|
| In vivo |
In conditional caALK2-transgenic mice with Ad.Cre on on postnatal day 7 (P7), LDN-193189 (3 mg/kg i.p) leads to mild calcifications surrounding the left tibia and fibula first visible at P13, and prevents radiographic lesions at P15 without causing weight loss or growth retardation, spontaneous fractures, decreased bone density or behavioral abnormalities. LDN193189 dorsalizes zebrafish embryos by inhibiting signaling pathways induced by bone morphogenetic protein (BMP)6 without effect on vascular development. In PCa-118b tumor-bearing mice, LDN-193189 treatment attenuates tumor growth and reduces bone formation in the tumors. In LDL receptor-deficient (LDLR-/-) mice, LDN-193189 potently inhibits development of atheroma. Moreover, LDN-193189 also exhibits the inhibitory effects on associated vascular inflammation, osteogenic activity, and calcification. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
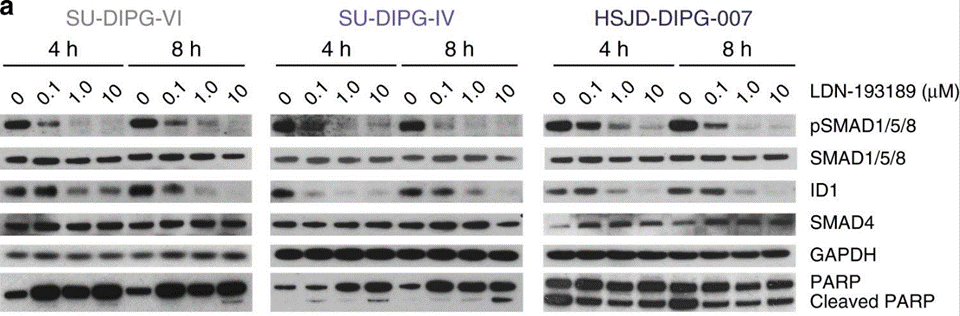
| Western blot | Smad / ID1 / PARP / Cleaved PARP pSmad |
|
31098401 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































