research use only
Erdafitinib (JNJ-42756493) FGFR inhibitor
Cat.No.S8401
Chemical Structure
Molecular Weight: 446.54
Quality Control
Products Often Used Together with Erdafitinib (JNJ-42756493)
| Related Targets | EGFR VEGFR JAK PDGFR Src HIF FLT FLT3 HER2 Bcr-Abl |
|---|---|
| Other FGFR Inhibitors | PD173074 AZD4547 (Fexagratinib) BLU9931 Futibatinib (TAS-120) LY2874455 PD-166866 H3B-6527 Zoligratinib (Debio-1347) Fisogatinib (BLU-554) SSR128129E |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| BA/F3 | Function assay | 24 hrs | Inhibition of FGFR3 (unknown origin) transfected in mouse BA/F3 cells assessed as decrease in cell proliferation in absence of mouse IL3 after 24 hrs by Alamar blue assay, IC50 = 0.01585 μM. | ChEMBL | ||
| BA/F3 | Function assay | 24 hrs | Inhibition of FGFR1 (unknown origin) transfected in mouse BA/F3 cells assessed as decrease in cell proliferation in absence of mouse IL3 after 24 hrs by Alamar blue assay, IC50 = 0.08318 μM. | ChEMBL | ||
| BA/F3 | Function assay | 24 hrs | Inhibition of VEGFR2 (unknown origin) transfected in mouse BA/F3 cells assessed as decrease in cell proliferation in absence of mouse IL3 after 24 hrs by Alamar blue assay, IC50 = 3.31131 μM. | ChEMBL | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 89 mg/mL
(199.31 mM)
Ethanol : 89 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 446.54 | Formula | C25H30N6O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1346242-81-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | JNJ-42756493 | Smiles | CC(C)NCCN(C1=CC2=NC(=CN=C2C=C1)C3=CN(N=C3)C)C4=CC(=CC(=C4)OC)OC | ||
Mechanism of Action
| Targets/IC50/Ki |
FGFR
RET
CSF-1R
PDGFR
Kit
VEGFR2
|
|---|---|
| In vitro |
JNJ-42756493 is a potent, oral pan-FGFR tyrosine kinase inhibitor with half-maximal inhibitory concentration values in the low nanomolar range for all members of the FGFR family (FGFR1 to FGFR4), with minimal activity on vascular endothelial growth factor receptor (VEGFR) kinases compared with FGFR kinases (approximately 20-fold potency difference). In vitro, the proliferation of cells treated with this compound is decreased, associated with increased apoptotic death and decreased cell survival. |
| In vivo |
In vivo, growth of NCI-H716 tumors is delayed by 5 days by drug treatment alone, although when drug delivery is stopped the relative tumor volume increased compared to control. This compound shows favorable drug like properties and displays a high distribution to lung, liver and kidney tissue. It is well tolerated at efficacious doses and results in potent dose-dependent antitumor activity accompanied by pharmacodynamic modulation of tumor FGFR and downstream pathway components. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | FGFR2 / p-FGFR PARP / Cleaved PARP / AKT / p-AKT / p-ERK / ERK2 |
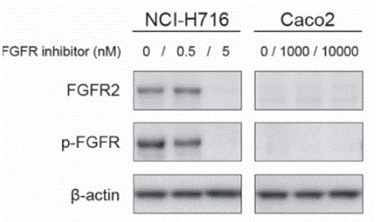
|
26675289 |
| Growth inhibition assay | Cell viability |
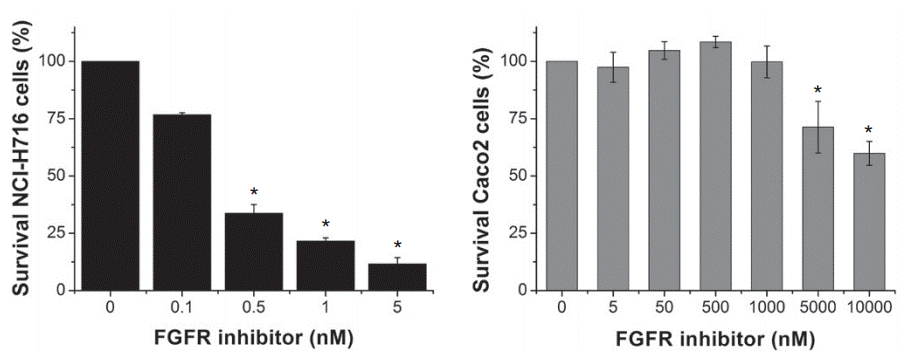
|
26675289 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05567185 | Recruiting | Urinary Bladder Neoplasms|Receptors Fibroblast Growth Factor |
Janssen Pharmaceutical K.K. |
March 3 2023 | Phase 1 |
| NCT05316155 | Recruiting | Urinary Bladder Neoplasms|Receptors Fibroblast Growth Factor |
Janssen Research & Development LLC |
April 11 2022 | Phase 1 |
| NCT05052372 | Terminated | Bladder Cancer|Urothelial Carcinoma|Transitional Cell Carcinoma|Platinum-Resistant Urothelial Carcinoma|Bladder Urothelial Carcinoma|FGFR Mutation|FGFR2 Gene Mutation|FGFR3 Gene Mutation|FGFR2 Amplification|Locally Advanced Urothelial Carcinoma|Metastatic Urothelial Carcinoma|Refractory Bladder Carcinoma|Refractory Bladder Urothelial Carcinoma |
xCures |
November 21 2021 | -- |
| NCT04330248 | Completed | Healthy |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. |
March 31 2020 | Phase 1 |
| NCT04083976 | Active not recruiting | Advanced Solid Tumor |
Janssen Research & Development LLC |
November 20 2019 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































