research use only
LY2874455 FGFR inhibitor
Cat.No.S7057
Chemical Structure
Molecular Weight: 444.31
Quality Control
| Related Targets | EGFR VEGFR PDGFR c-Met Src MEK CSF-1R FLT3 HER2 c-Kit |
|---|---|
| Other FGFR Inhibitors | PD173074 AZD4547 (Fexagratinib) BLU9931 Futibatinib (TAS-120) PD-166866 H3B-6527 Zoligratinib (Debio-1347) Fisogatinib (BLU-554) SSR128129E Ferulic Acid |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| NCI-H1581 | Antiproliferative assay | 72 hrs | Antiproliferative activity against FGFR1-amplified human NCI-H1581 cells after 72 hrs by CCK8/MTT assay, IC50<0.0005μM | 27348537 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 88 mg/mL
(198.05 mM)
Ethanol : 88 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 444.31 | Formula | C21H19Cl2N5O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1254473-64-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CC(C1=C(C=NC=C1Cl)Cl)OC2=CC3=C(C=C2)NN=C3C=CC4=CN(N=C4)CCO | ||
Mechanism of Action
| Targets/IC50/Ki |
FGFR2
(Cell-free assay) 2.6 nM
FGFR1
(Cell-free assay) 2.8 nM
FGFR4
(Cell-free assay) 6 nM
FGFR3
(Cell-free assay) 6.4 nM
VEGFR2
(Cell-free assay) 7 nM
|
|---|---|
| In vitro |
In RT-112 cells, HUVECs, KATO-III cells, and SNU-16 cells, LY2874455 inhibits FGF/FGFR-mediated signaling activities. This compound shows FGFR-dependent antiproliferative effects in KMS-11, OPM-2, SNU-16 and KATO-III cells.
|
| Kinase Assay |
Biochemical filter-binding assays for detection of FGFR phosphorylation activities
|
|
Reaction mixtures contains 8 mM Tris-HCl (pH 7.5), 10 mmol/L HEPES, 5 mM dithiothreitol, 10 μM ATP, 0.5 μCi 33P-ATP, 10 mM MnCl2, 150 mM NaCl, 0.01% Triton X-100, 4% dimethyl sulfoxide, 0.05 mg/mL poly(Glu:Tyr) (4:1, average molecular weight of 20–50 kDa), and 7.5, 7.5, and 16 ng of FGFR1, FGFR3, and FGFR4, respectively, and are incubated at room temperature for 30 minutes followed by termination with 10% H3PO4. The reaction mixtures are transferred to 96-well MAFB filter plates that are washed 3 times with 0.5% H3PO4. After air-drying, the plates are read with a Trilux reader.
|
|
| In vivo |
LY2874455 exhibits a potent inhibition of FGF-induced Erk phosphorylation in the heart tissues of mice with TED50 and TED90 values of 1.3 and 3.2 mg/kg, respectively. In mice bearing RT-112, OPM-2 (DSMZ), SNU-16, or NCI-H460 xenograft, this compound (3 mg/kg p.o.) results in dose-dependent inhibition of the tumor growth.
|
References |
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | p-FRS2 / FRS2 / p-AKT / AKT / p-ERK / ERK / p-PLCγ1 / PLCγ1 MCL-1 |
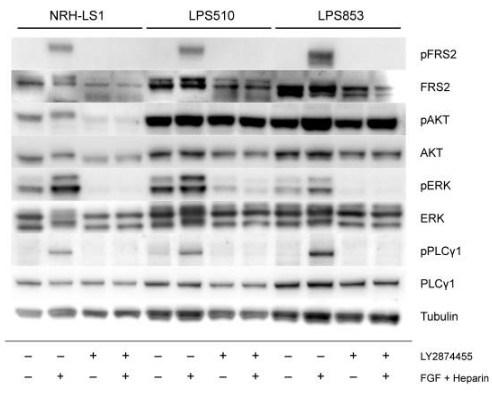
|
30795553 |
| Growth inhibition assay | Cell death |
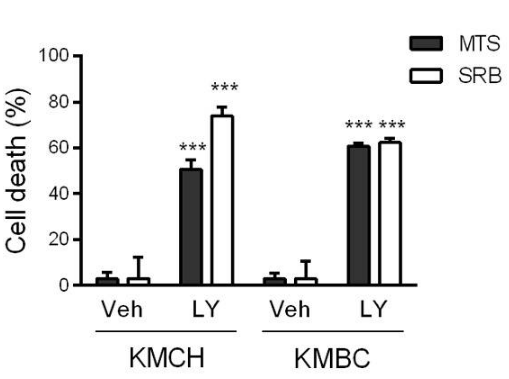
|
29408314 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03125239 | Completed | Relapsed Adult Acute Myeloid Leukemia|Refractory Adult Acute Myeloid Leukemia |
Jacqueline Garcia MD|Eli Lilly and Company|Dana-Farber Cancer Institute |
August 10 2017 | Phase 1 |
| NCT01212107 | Completed | Advanced Cancer |
Eli Lilly and Company |
December 2010 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































