research use only
Zoligratinib (Debio-1347) FGFR inhibitor
Cat.No.S7665
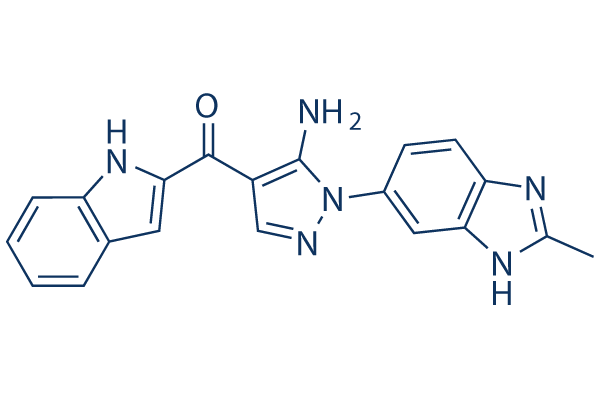
Chemical Structure
Molecular Weight: 356.38
Quality Control
| Related Targets | EGFR VEGFR JAK PDGFR Src HIF FLT FLT3 HER2 Bcr-Abl |
|---|---|
| Other FGFR Inhibitors | PD173074 AZD4547 (Fexagratinib) BLU9931 Futibatinib (TAS-120) LY2874455 PD-166866 H3B-6527 Fisogatinib (BLU-554) SSR128129E Ferulic Acid |
Solubility
|
In vitro |
DMSO
: 71 mg/mL
(199.22 mM)
Ethanol : 1 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 356.38 | Formula | C20H16N6O |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1265229-25-1 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | CH5183284, FF284 | Smiles | CC1=NC2=C(N1)C=C(C=C2)N3C(=C(C=N3)C(=O)C4=CC5=CC=CC=C5N4)N | ||
Mechanism of Action
| Targets/IC50/Ki |
FGFR2
(Cell-free assay) 7.6 nM
FGFR1
(Cell-free assay) 9.3 nM
FGFR3
(Cell-free assay) 22 nM
FGFR4
(Cell-free assay) 290 nM
|
|---|---|
| In vitro |
In cell-based assay, CH5183284 prevents autophosphorylation of FGFR1, FGFR2, and FGFR3 at 100 to 300 nM in the DMS114 (FGFR1 amplification), SNU-16 (FGFR2 amplification), and KMS11 [t(4;14) translocation and FGFR3 Y373C mutation] cell lines. CH5183284 thus produces selective antiproliferative activity against cancer cell lines harboring genetic alterations in FGFR. CH5183284 also inhibit FGFR2 harboring one type of the gatekeeper mutation (V564F) that causes resistance to other FGFR inhibitors.
|
| Kinase Assay |
Protein kinase assay
|
|
The inhibitory activity of CH5183284/Debio 1347 against FGFR1 is evaluated using a radiometric filter assay by measuring the incorporation of 33Pi with a microplate scintillation counter. The phosphorylation activities of LCK, EGFR, KIT, MET, SRC, BRK, FGFR2, Flt3, LTK, INSR, YES, ABL, EPHA2, ZAP70, Fyn, IGF1R, KDR, and PDGFR on substrate peptides are determined by homogeneous time-resolved fluorescence assay with LANCE Eu-W1024 labeled anti-phosphotyrosine PT66 antibody according to standard methods. Time-resolved fluorescence is measured with an EnVision HTS microplate reader. The activities of Aurora A, Akt1/PKBα, PKA, Cdk1/cyclin B, Cdk2/cyclin A, PKCα, PKCβ1 and PKCβ2 on substrate peptides are determined by IMAP FP Screening Express Progressive Binding System. Fluorescence polarization is measured with an EnVision HTS microplate reader.
|
|
| In vivo |
CH5183284 (100 mg/kg/day, p.o.) shows selective and significant antitumor activity against xenografts with FGFR genetic alterations such as KG1 (leukemia, FGFR1OP-FGFR1 fusion), SNU-16 (gastric cancer, FGFR2 amplification), MFE-280 (endometrial cancer, FGFR2 S252W mutation), UM-UC-14 (bladder cancer, FGFR3 S249C mutation), and RT112/84 (bladder cancer, FGFR3-TACC3 fusion).
|
References |
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | pERK / ERK / pAKT / AKT DUSP6 pY-FGFR / pY-KDR / pY-PDGFR / pY-c-KIT |
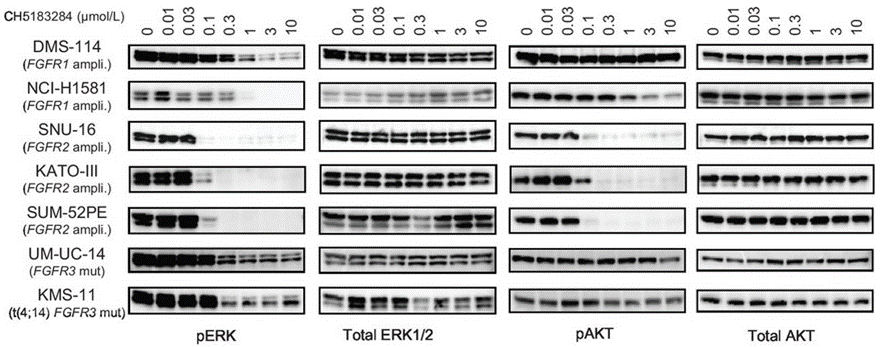
|
26438159 |
| Growth inhibition assay | Cell viability |
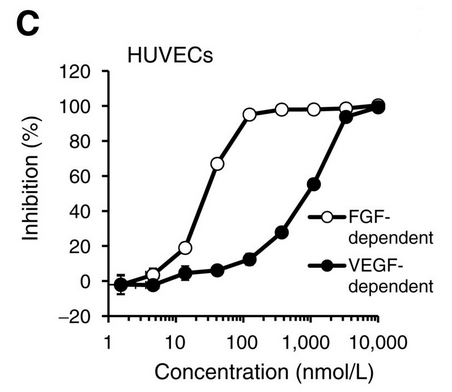
|
25169980 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03834220 | Terminated | Solid Tumor |
Debiopharm International SA|Caris Life Sciences|Optimal Research (Just In Time sites) |
March 22 2019 | Phase 2 |
| NCT01948297 | Terminated | Solid Tumours |
Debiopharm International SA |
August 2013 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































