research use only
AZD1390 ATM inhibitor
Cat.No.S8680
Chemical Structure
Molecular Weight: 477.57
Quality Control
| Related Targets | PI3K Akt mTOR GSK-3 DNA-PK AMPK PDPK1 PTEN PP2A PDK |
|---|---|
| Other ATM/ATR Inhibitors | KU-60019 Berzosertib (VE-822) Ceralasertib (AZD6738) Camonsertib (RP-3500) Lartesertib (M4076) KU-55933 VE-821 AZ20 AZD0156 Mirin |
Solubility
|
In vitro |
Ethanol : 95 mg/mL
DMSO
: 14 mg/mL
(29.31 mM)
Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 477.57 | Formula | C27H32FN5O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 2089288-03-7 | -- | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CC(C)N1C2=C(C=NC3=CC(=C(C=C32)C4=CN=C(C=C4)OCCCN5CCCCC5)F)N(C1=O)C | ||
Mechanism of Action
| Targets/IC50/Ki |
ATM
(Cell-based assay) 0.78 nM
|
|---|---|
| In vitro |
AZD1390 blocks ATM-dependent DDR (DNA damage response) pathway activity and combines with radiation to induce G2 cell cycle phase accumulation, micronuclei, and apoptosis. This compound radiosensitizes glioma and lung cancer cell lines, with p53 mutant glioma cells generally being more radiosensitized than wild type. It results in increased genome instability.
|
| In vivo |
AZD1390 displays excellent oral bioavailability in preclinical species (66% in rat and 74% in dog). It can efficiently cross the BBB in non-human primate PET studies. Profound tumor regressions and increased animal survival (>50 days) have been observed in orthotopic xenograft models of brain cancer following just 2 or 4 days combination treatment of this compound with radiotherapy, compared to radiotherapy treatment alone. In in vivo syngeneic and patient-derived glioma as well as orthotopic lung-brain metastatic models, this compound dosed in combination with daily fractions of IR (whole-brain or stereotactic radiotherapy) significantly induces tumor regressions and increased animal survival compared to IR treatment alone. This compound has favorable physical, chemical, PK, and PD properties suitable for clinical applications that require exposures within the central nervous system.
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | pChk2 pATM (S1981) / ATM / pKAP1(S824) / KAP1 / pCDK1 |
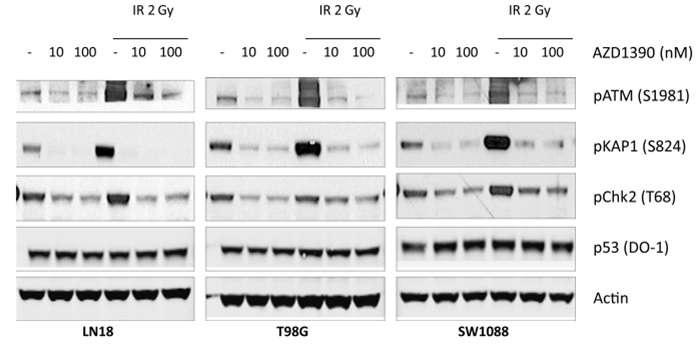
|
29938225 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05182905 | Recruiting | Glioblastoma|Glioma|Glioblastoma Multiforme|Glioma Malignant |
Nader Sanai|Barrow Neurological Institute|Ivy Brain Tumor Center|AstraZeneca|St. Joseph''s Hospital and Medical Center Phoenix |
March 9 2022 | Early Phase 1 |
| NCT03423628 | Recruiting | Recurrent Glioblastoma Multiforme|Primary Glioblastoma Multiforme|Brain Neoplasms Malignant|Leptomeningeal Disease (LMD) |
AstraZeneca |
April 2 2018 | Phase 1 |
| NCT03215381 | Completed | Healthy Volunteer Male Subjects |
AstraZeneca|Karolinska Institutet Quintiles IMS |
October 10 2017 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































