research use only
Metronidazole Antineoplastic and Immunosuppressive Antibiotics inhibitor
Cat.No.S1907
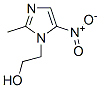
Chemical Structure
Molecular Weight: 171.15
Quality Control
| Related Targets | HDAC PARP ATM/ATR DNA-PK WRN DNA/RNA Synthesis Topoisomerase PPAR Sirtuin Casein Kinase |
|---|---|
| Other Antineoplastic and Immunosuppressive Antibiotics Inhibitors | Staurosporine (STS) Cyclosporin A Puromycin Dihydrochloride Oligomycin A (MCH 32) Geldanamycin (NSC 122750) Honokiol Streptozotocin (STZ) Cephalomannine Sodium Monensin (NSC 343257) 10-Deacetylbaccatin-III |
Solubility
|
In vitro |
DMSO
: 34 mg/mL
(198.65 mM)
Water : 12 mg/mL (Warmed with 50℃ water bath; Ultrasonicated) Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 171.15 | Formula | C6H9N3O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 443-48-1 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CC1=NC=C(N1CCO)[N+](=O)[O-] | ||
Mechanism of Action
| Targets/IC50/Ki |
DNA synthesis
|
|---|---|
| In vitro |
Metronidazole is relatively inactive until it is metabolized within host or microbial cells. It is activated when it receives an electron from ferredoxin or flavodoxin that is reduced by POR in anaerobic or microaerophilic bacteria or luminal parasites. This compound damages cells by forming protein and DNA adducts. It has activity against protozoans like Entamoeba histolytica, Giardia lamblia and Trichomonas vaginalis, for which the drug is first approved as an effective treatment. The activity of this chemical against anaerobic bowel flora has been used for prophylaxis and treatment of patients with Crohn's disease who might develop an infectious complication. It has played an important role in anaerobic-related infections. This compound has notable effectiveness in treating anaerobic brain abscesses. Its resistance tends to result from de novo mutation in the resident rdxA gene, rather than from lateral transfer of mutant rdxA (or other) genes from unrelated but Mtzr strains. It partially inhibits growth stimulate forward mutation to rifampin resistance in rdxA(+) (Metronidazole(s)) and also in rdxA (Metronidazole(r)) H. pylori strains, and that expression of rdxA in Escherichia coli results in equivalent Mtz-induced mutation. This compound leads to apoptosis-like features in growing cultures of axenic B. hominis, including key morphological and biochemical features of programmed cell death (PCD), viz. nuclear condensation and nicked DNA in nucleus, reduced cytoplasmic volume, externalization of phosphatidylserine and maintenance of plasma membrane integrity with increasing permeability.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06387147 | Not yet recruiting | Acute Mesenteric Ischemia |
Assistance Publique - Hôpitaux de Paris |
September 1 2024 | Phase 3 |
| NCT05748145 | Recruiting | Colorectal Cancer |
Oncology Institute of Southern Switzerland |
September 11 2023 | Phase 2 |
| NCT04273997 | Unknown status | Pilonidal Sinus |
S.L.A. Pharma AG |
October 1 2021 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































