- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Roxadustat (FG-4592) HIF Proly Hydroxylase inhibitor
Cat.No.S1007
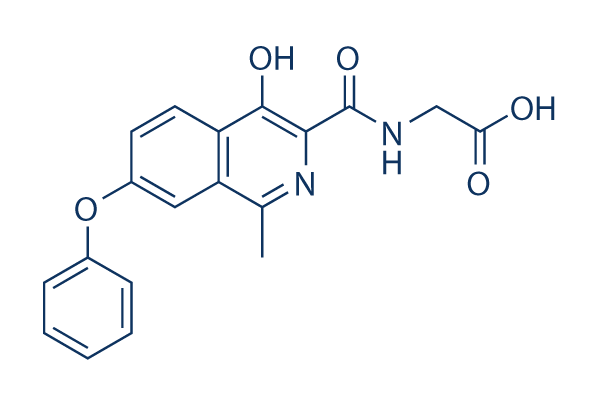
Chemical Structure
Molecular Weight: 352.34
Jump to
Quality Control
Batch:
Purity:
99.96%
99.96
| Related Targets | EGFR VEGFR JAK PDGFR FGFR Src FLT FLT3 HER2 Bcr-Abl |
|---|---|
| Other HIF Products | PT2399 PX-478 Dihydrochloride BAY 87-2243 KC7F2 Lificiguat (YC-1) IOX2 CAY10585 (LW 6) Molidustat (BAY 85-3934) PT2385 IDF-11774 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| EpSC cell | Cell viability assay | 5 µM or 10 µM | 24 h | FG-4592 increased EpSC cell viability in normoxia. | 29742498 | |
| Adiposederived mesenchymal stem cells (ADMSCs) | Function assay | 5 μM | the level of HIF-1a expression was much higher in the FG-4592-treated group than in the vehicletreated group. | 27021233 | ||
| Hep3B | Function assay | 50 to 250 uM | 10 hrs | Inhibition of HIF-PHD2 in human Hep3B cells assessed as HIF-1alpha protein stabilization at 50 to 250 uM after 10 hrs by Western blot method | 29856623 | |
| Hep3B | Function assay | 50 to 250 uM | 10 hrs | Inhibition of HIF-PHD2 in human Hep3B cells assessed as HIF-2alpha protein stabilization at 50 to 250 uM after 10 hrs by Western blot method | 29856623 | |
| Hep3B | Function assay | 50 uM | 10 hrs | Inhibition of HIF-PHD2 in human Hep3B cells assessed as upregulation of EPO mRNA level at 50 uM after 10 hrs by RT-PCR method | 29856623 | |
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 70 mg/mL
(198.67 mM)
Ethanol : 2 mg/mL Water : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 352.34 | Formula | C19H16N2O5 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 808118-40-3 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | ASP1517 | Smiles | CC1=C2C=C(C=CC2=C(C(=N1)C(=O)NCC(=O)O)O)OC3=CC=CC=C3 | ||
Mechanism of Action
| In vitro |
Roxadustat (FG-4592) is an oral inhibitor of hypoxia inducible factor (HIF) prolyl hydroxylase currently in clinical development for the treatment of anemia. It stabilizes the activities of HIF, a cytosolic transcription factor, leading to activation of the genes associated with erythropoiesis, including erythropoietin and enzymes involved in iron metabolism. |
|---|---|
| In vivo |
Roxadustat (FG-4592) (50 mg/kg; i.p.; daily for 7 days) protects the survival of motor neurons and improves recovery from spinal cord injury. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
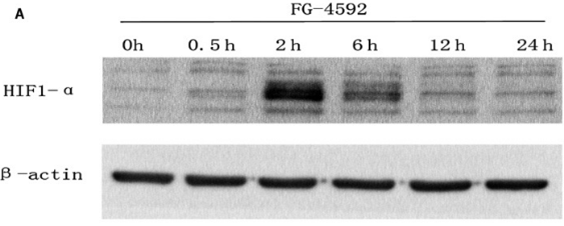
| Western blot | HIF-α CA9 / NDRG1 / GLUT1 / GLUT3 / HK2 / PHD2 / JMJD1A / JMJD2B / JMJD2C |
|
30334352 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03303066 | Completed | Anemia|Myelodysplastic Syndrome |
FibroGen|AstraZeneca |
June 6 2018 | Phase 2|Phase 3 |
| NCT02988973 | Completed | Chronic Kidney Disease |
Astellas Pharma Inc|FibroGen |
January 12 2017 | Phase 3 |
| NCT02964936 | Completed | Chronic Kidney Disease |
Astellas Pharma Inc|FibroGen |
January 11 2017 | Phase 3 |
| NCT02952040 | Completed | Healthy Subjects |
Astellas Pharma Inc|FibroGen |
November 2016 | Phase 1 |
| NCT02952092 | Completed | Hemodialysis Chronic Kidney Disease Patients With Anemia |
Astellas Pharma Inc|FibroGen |
November 30 2016 | Phase 3 |
| NCT02805374 | Completed | Healthy Subjects |
Astellas Pharma Inc|FibroGen |
July 2016 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
Can you tell me what the half-life of it is, please?
Answer:
Its half-life is around 12 hours. Please see the reference below: https://www.ncbi.nlm.nih.gov/pubmed/26846333






































