- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Molidustat (BAY 85-3934) HIF modulator
Cat.No.S8138
-chemical-structure-s8138.gif)
Chemical Structure
Molecular Weight: 314.30
Jump to
Quality Control
Batch:
Purity:
99.82%
99.82
| Related Targets | EGFR VEGFR JAK PDGFR FGFR Src FLT FLT3 HER2 Bcr-Abl |
|---|---|
| Other HIF Inhibitors | PT2399 PX-478 Dihydrochloride BAY 87-2243 KC7F2 Lificiguat (YC-1) IOX2 CAY10585 (LW 6) PT2385 IDF-11774 MK-8617 |
Solubility
|
In vitro |
DMSO
: 10 mg/mL
(31.81 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 314.30 | Formula | C13H14N8O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1154028-82-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | C1COCCN1C2=NC=NC(=C2)N3C(=O)C(=CN3)N4C=CN=N4 | ||
Mechanism of Action
| Targets/IC50/Ki |
PHD2
(Cell-free assay) 280 nM
PHD3
(Cell-free assay) 450 nM
PHD1
(Cell-free assay) 480 nM
|
|---|---|
| In vitro |
In vitro, Molidustat (BAY 85-3934) induces the transcription of hypoxia-sensitive genes in HeLa, A549, and Hep3B cells. |
| Kinase Assay |
Prolyl hydroxylase assay
|
|
Biotinylated HIF-1α 556–574 (biotinyl-DLDLEMLAPYIPMDDDFQL) is bound to white 96-well NeutrAvidin high binding capacity plates, which are pre-blocked with Blocker Casein and subsequently blocked with 1 mM biotin. The immobilized peptide substrate is incubated with the appropriate amount of HIF-PH in buffer containing 20 mM Tris (pH 7.5), 5 mM KCl, 1.5 mM MgCl2, 20 µM 2-oxoglutarate, 10 µM FeSO4, 2 mM ascorbate, 4% protease inhibitors without EDTA in a final volume of 100 µl, with or without Molidustat (BAY 85-3934) added at appropriate concentrations. The reaction time is 60 min. To stop the reaction, plates are washed three times with wash buffer. Hydroxylated biotinyl-HIF-1α 556–574 is incubated with Eu-VBC in 100 µl binding buffer (50 mM Tris [pH 7.5], 120 mM NaCl) for 60 min at room temperature. After washing six times with DELFIA wash buffer and adding 100 µl enhancer solution, the amount of bound VBC is determined by measuring time-resolved fluorescence with a Tecan infinite M200 plate reader. Measurements are taken in triplicate or more, and results are expressed as means ± SEM. IC50 values for this compound are determined after curve fitting using GraphPad Prism software applying the four-parameter logistic equation to the data sets.
|
|
| In vivo |
In healthy Wistar rats and cynomolgus monkeys, Molidustat (BAY 85-3934) (1.5 mg/kg, p.o.) induces dose-dependent production of EPO and erythropoiesis. In rats with induced renal anemiabe, this compound (5 mg/kg, p.o.) is also effective in raising hematocrit levels while stimulating endogenous EPO production without hypertensive effects. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | HIF-1α |
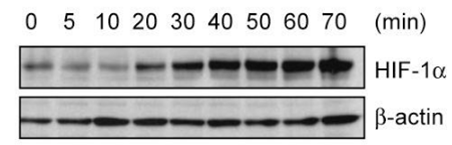
|
25392999 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03418168 | Completed | Anemia|Renal Insufficiency Chronic |
Bayer |
February 22 2018 | Phase 3 |
| NCT03351166 | Completed | Anemia|Renal Insufficiency Chronic |
Bayer |
January 22 2018 | Phase 3 |
| NCT03350347 | Completed | Anemia|Renal Insufficiency Chronic |
Bayer |
December 13 2017 | Phase 3 |
| NCT02312973 | Completed | Renal Insufficiency Chronic |
Bayer |
January 14 2015 | Phase 1 |
| NCT01458028 | Completed | Anemia |
Bayer |
September 2011 | Phase 1 |
| NCT01332942 | Completed | Anemia |
Bayer |
April 2011 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































