research use only
Clindamycin Bacterial inhibitor
Cat.No.S2830
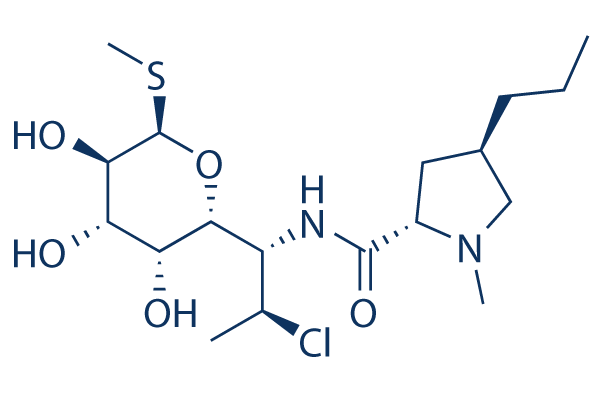
Chemical Structure
Molecular Weight: 424.98
Quality Control
| Related Targets | Integrase Antibiotics Anti-infection Fungal Antiviral COVID-19 Parasite Reverse Transcriptase HIV HCV Protease |
|---|---|
| Other Bacterial Inhibitors | Berberine BTZ043 Racemate Teicoplanin Pefloxacin Mesylate Ornidazole Proanthocyanidins Solithromycin Skatole Berberine Sulfate Furagin |
Solubility
|
In vitro |
DMSO
: 85 mg/mL
(200.0 mM)
Ethanol : 85 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 424.98 | Formula | C18H33ClN2O5S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 18323-44-9 | -- | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CCCC1CC(N(C1)C)C(=O)NC(C2C(C(C(C(O2)SC)O)O)O)C(C)Cl | ||
Mechanism of Action
| In vitro |
Clindamycin is a semisynthetic analogue of lincomycin. It primarily inhibits the initiation of peptide chain synthesis by deregulating enzyme-catalyzed initiation of peptide bounds. This compound appears to have a modest effect on protein synthesis by certain mammalian cells. It is active against most gram-positive aerobic bacteria. This antibiotic is about eight times more active than lincomycin against Staphylococcus aureus and Streptococcus pneumonia. It is four times more active than erythromycin against S. aureus and is active even against strains that are resistant to erythromycin, penicillin, and methicillin. It is active against gram-positive anaerobes. This agent highly activates against Bacteroides specie. It also alters the bacterial surface in such a way that phagocytosis and intracellular killing of the bacteria is greatly facilitated. This drug potentiates opsonization and phagocytosis.
|
|---|---|
| In vivo |
Clindamycin (50mg/kg daily administrated by intramuscular) increases the survival rate of monkeys infected with penicillin-resistant S. aureu to 87.5% (7/8). This compound (40 mg/kg administrated three times daily) protects 87.5% (7/8) of rabbits from anaerobic pulmonary infections induced by transtracheal inoculation of a mixture of B. fragillis, Streptococcus morbillorm, Fusobacterium nucleatum and Eubacterium lentum. This chemical (400 mg/kg treated by mixed in the diet) increases survival rate of mice infected with the Toxoplasma gondii to 100%, while all animals die in untreated group.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05926869 | Completed | Acne Vulgaris |
Jinnah Postgraduate Medical Centre |
August 1 2022 | Phase 2 |
| NCT05223400 | Completed | Infection Bacterial |
Alexandria University |
March 1 2022 | -- |
| NCT04946500 | Unknown status | Prosthetic Joint Infection|Staphylococcus |
University Hospital Brest |
May 15 2021 | -- |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































