- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Cabazitaxel Microtubule Associated inhibitor
Cat.No.S3022
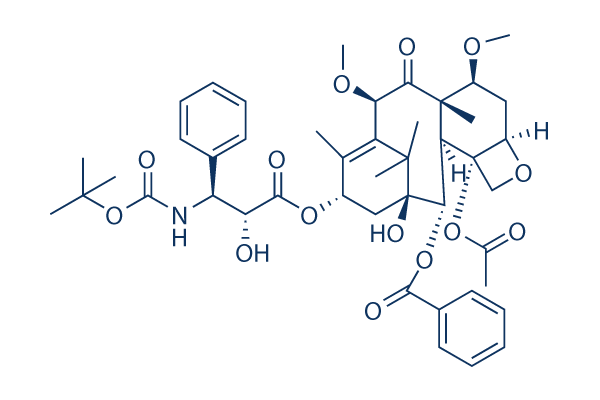
Chemical Structure
Molecular Weight: 835.93
Jump to
Quality Control
Batch:
Purity:
99.99%
99.99
| Related Targets | Akt Wnt/beta-catenin HSP PKC ROCK Integrin Bcr-Abl Actin FAK Kinesin |
|---|---|
| Other Microtubule Associated Products | Nocodazole MMAF Patupilone (Epothilone B) Lexibulin (CYT997) CW069 Combretastatin A4 ABT-751 (E7010) Epothilone A Cucurbitacin B TAI-1 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| SGC7901 | Growth inhibition assay | Growth inhibition of human SGC7901 cells by MTT assay, GI50=0.0003553μM | 24405702 | |||
| U937 | Growth inhibition assay | Growth inhibition of human U937 cells by MTT assay, GI50=0.0005391μM | 24405702 | |||
| MCF7 | Growth inhibition assay | Growth inhibition of human MCF7 cells by MTT assay, GI50=0.001187μM | 24405702 | |||
| PANC1 | Growth inhibition assay | Growth inhibition of human PANC1 cells by MTT assay, GI50=0.001283μM | 24405702 | |||
| HT1080 | Growth inhibition assay | Growth inhibition of human HT1080 cells by MTT assay, GI50=0.001406μM | 24405702 | |||
| DU145 | Growth inhibition assay | Growth inhibition of human DU145 cells by MTT assay, GI50=0.001429μM | 24405702 | |||
| A549 | Growth inhibition assay | Growth inhibition of human A549 cells by MTT assay, GI50=0.001483μM | 24405702 | |||
| A431 | Growth inhibition assay | Growth inhibition of human A431 cells by MTT assay, GI50=0.001483μM | 24405702 | |||
| HeLa | Growth inhibition assay | Growth inhibition of human HeLa cells by MTT assay, GI50=0.001799μM | 24405702 | |||
| K562 | Growth inhibition assay | Growth inhibition of human K562 cells by MTT assay, GI50=0.004186μM | 24405702 | |||
| HL60 | Growth inhibition assay | Growth inhibition of human HL60 cells by MTT assay, GI50=0.004736μM | 24405702 | |||
| BGC823 | Growth inhibition assay | Growth inhibition of human BGC823 cells by MTT assay, GI50=0.4672μM | 24405702 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells, IC50=0.00148μM | 28850227 | |||
| MES-SA/Dx5 | Growth inhibition assay | 72 hrs | Growth inhibition of human MES-SA/Dx5 cells after 72 hrs by SRB assay, IC50=0.015μM | 29251920 | ||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for TC32 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for A673 cells) | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SK-N-MC cells | 29435139 | |||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for TC32 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for MG 63 (6-TG R) cells | 29435139 | |||
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for U-2 OS cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh41 cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Saos-2 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for OHS-50 cells | 29435139 | |||
| NCI-H524 | Cytotoxicity assay | 2 hrs | Cytotoxicity in human NCI-H524 cells pre-incubated for 2 hrs followed by compound wash out and subsequently incubated for 70 hrs by Cell Titer Glo assay, IC50=0.00026μM | 30735385 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 167 mg/mL
(199.77 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 835.93 | Formula | C45H57NO14 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 183133-96-2 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | XRP6258, RPR-116258A, TXD 258, Taxoid XRP6258 | Smiles | CC1=C2C(C(=O)C3(C(CC4C(C3C(C(C2(C)C)(CC1OC(=O)C(C(C5=CC=CC=C5)NC(=O)OC(C)(C)C)O)O)OC(=O)C6=CC=CC=C6)(CO4)OC(=O)C)OC)C)OC | ||
Mechanism of Action
| Features |
A semi-synthetic derivative of a natural taxoid.
|
|---|---|
| Targets/IC50/Ki |
Microtubule
(Cell-free assay) |
| In vitro |
Cabazitaxel increases CYP3A enzyme activities in rat hepatocytes. The mean ex-vivo human plasma protein binding of this compound is 91.6%. It is rapidly and extensively metabolised in numerous metabolites. This compound demonstrates activity in several murine and human resistant cell lines. With a 4-day exposure to this chemical, cytotoxicity is noted with relatively low cabazitaxel concentrations. It shows high antitumor activity in 3 human colorectal cell lines (HCT-116, HCT-8, and HT-29). |
| In vivo |
In accompanying models, Cabazitaxel is noted to have significant antitumor activity. In murine tumor xenografts (colon C38 and pancreas P03), this compound elicites complete tumor regressions. Using SF-295 and U251 human glioblastoma cell lines, both orthotopic and subcutaneous murine xenografts are generated. This chemical treatment leads to complete regression in the majority of subcutaneously implanted tumors. Furthermore, in orthotopic models, it leads to complete tumor regression in 4 out of 10 U251 tumors. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04622761 | Not yet recruiting | Prostate Cancer |
The Clatterbridge Cancer Centre NHS Foundation Trust|University of Liverpool |
January 15 2021 | Phase 2 |
| NCT04495179 | Completed | Progressive Metastatic Castrate-Resistant Prostate Cancer |
AstraZeneca|Parexel |
August 4 2020 | Phase 2 |
| NCT03257891 | Unknown status | Adrenocortical Carcinoma |
Azienda Socio Sanitaria Territoriale degli Spedali Civili di Brescia|San Luigi Gonzaga Hospital |
January 25 2018 | Phase 2 |
| NCT03043989 | Terminated | Prostate Cancer |
Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins|Maryland Technology Development Corporation |
March 21 2017 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
What is the elimination half-life of this compound?
Answer:
According to the paper report, its elimination half-life is 95h.






































