research use only
Ciclopirox ATPase inhibitor
Cat.No.S2528
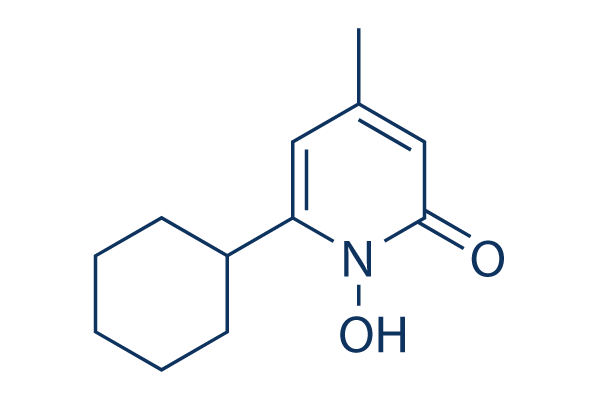
Chemical Structure
Molecular Weight: 207.27
Quality Control
| Related Targets | CFTR CRM1 CD markers AChR Calcium Channel Sodium Channel Potassium Channel GABA Receptor TRP Channel GluR |
|---|---|
| Other ATPase Inhibitors | (-)-Blebbistatin Thapsigargin Brefeldin A (BFA chemical) CB-5083 Golgicide A Sodium orthovanadate Oleic Acid Bufalin CDN1163 Ginsenoside Rb1 |
Solubility
|
In vitro |
DMSO
: 41 mg/mL
(197.8 mM)
Ethanol : 41 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 207.27 | Formula | C12H17NO2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 29342-05-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | HOE 296b, Penlac | Smiles | CC1=CC(=O)N(C(=C1)C2CCCCC2)O | ||
Mechanism of Action
| Targets/IC50/Ki |
ATPase
|
|---|---|
| In vitro |
Ciclopirox olamine (CPX) is a lipophilic bidentate iron chelator that stabilizes HIF-1alpha under normoxic conditions at lower concentrations than other iron chelators, probably by inhibiting HIF-1alpha hydroxylation. This compound-induced HIF-1 mediates reporter gene activity and endogenous HIF-1 target gene expression, including elevation of transcription, mRNA, and protein levels of the vascular endothelial growth factor (VEGF). It inhibits growth of C. albicans yeast and hyphal cells in a dose-dependent manner. This chemical blocks H2O2-induced mitochondrial injury by maintaining mitochondrial transmembrane potential (Deltapsim). It completely blocks H2O2-stimulated release of lactate dehydrogenase (a marker of cell death) and decreases in MTT reduction (a marker of mitochondrial function) in adenocarcinoma SK-HEP-1 cells. This compound effectively inhibits H2O2-induced mitochondrial permeability transition pore (MPTP) opening. It increases the MTP, maintained it high, and blocks the ATP depletion in glucose-deprived SIN-1-treated astrocytes. This chemical protects astrocytes from peroxynitritecytotoxicity by attenuating peroxynitrite-induced mitochondrial dysfunction. It is a substituted pyridone antimycotic drug, unrelated to the imidazole derivatives and its topical application ensures maximum local bioavailability. This compound acts on fungi by inhibiting the intracellular uptake of essential substrates and ions and this probably acts on the Candida ability to express its adherence mechanisms. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT00990587 | Completed | Hematologic Malignancy|Acute Lymphocytic Leukemia|Chronic Lymphocytic Leukemia|Myelodysplasia|Acute Myeloid Leukemia|Chronic Myelogenous Leukemia|Hodgkin''s Disease |
University Health Network Toronto|The Leukemia and Lymphoma Society |
October 2009 | Phase 1 |
| NCT01646580 | Terminated | Dermatomycoses |
Ferrer Internacional S.A. |
October 2008 | Phase 4 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































