- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Omecamtiv mecarbil (CK-1827452) Cardiac Myosin Activator
Cat.No.S2623
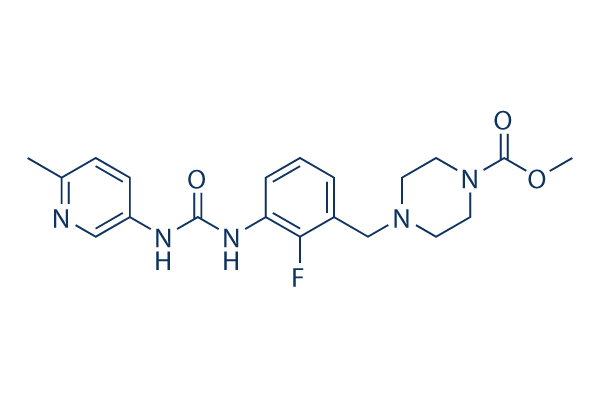
Chemical Structure
Molecular Weight: 401.43
Jump to
Quality Control
Batch:
Purity:
99.66%
99.66
Solubility
|
In vitro |
DMSO
: 80 mg/mL
(199.28 mM)
Ethanol : 6 mg/mL Water : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 401.43 | Formula | C20H24FN5O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 873697-71-3 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CC1=NC=C(C=C1)NC(=O)NC2=CC=CC(=C2F)CN3CCN(CC3)C(=O)OC | ||
Mechanism of Action
| Targets/IC50/Ki |
Myosin ATPase
|
|---|---|
| In vitro |
In vitro, Omecamtiv mecarbil (CK-1827452) selectively activates cardiac myosin by increasing the myosin ATPase rate. In isolated cardiac myocytes, it results in increase of myocyte contractility and overcomes of the myosin inhibitor BDM without increasing the calcium transient or inhibiting the PDE pathway.
|
| In vivo |
Omecamtiv mecarbil (CK-1827452) significantly increases fractional shortening starting at 0.4 mM plasma concentrations in SD rats, sham animals and in rats with heart failure. In conscious dogs with myocardial infarction (MI-sHF), it leads to a significant increase in wall thickening (25%), stroke volume (44%), cardiac output (22%) and left ventricular (LV) systolic ejection time (26%). In addition, this compound also results in the decreases of some hemodynamic parameters including heart rate, mean left atrial pressure, and LV end-diastolic pressure. In conscious dogs with left ventricular hypertrophy (LVH-sHF), it leads to similar and not statistically different effects on hemodynamic parameters.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04464525 | Withdrawn | Chronic Heart Failure With Reduced Ejection Fraction |
Cytokinetics |
December 18 2020 | Phase 3 |
| NCT02601001 | Completed | Healthy Volunteer |
Cytokinetics |
November 13 2015 | Phase 1 |
| NCT01300013 | Completed | Heart Failure |
Cytokinetics |
April 2011 | Phase 2 |
| NCT01077167 | Withdrawn | Heart Failure |
Cytokinetics |
July 2010 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
We would like to know its half-life, could you please provide some information?
Answer:
In a press release from cytokinetics (http://www.cytokinetics.com/press_releases/release/pr_1133989715), the company claimed that it has a sufficiently long half-life to support oral dosing. In a later press release (http://www.cytokinetics.com/press_releases/release/pr_1213309188), cytokinetics updated clinical trail result and mentioned that its elimination half-life is 22 hours.






































