research use only
Plerixafor (AMD3100) 8HCl CXCR antagonist
Cat.No.S3013
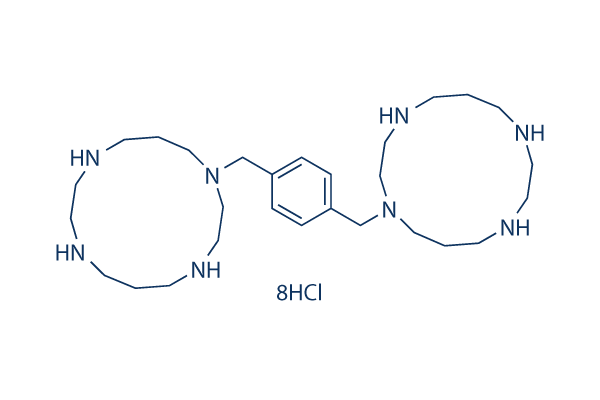
Chemical Structure
Molecular Weight: 794.47
Quality Control
| Related Targets | Hedgehog/Smoothened PKA Adrenergic Receptor AChR 5-HT Receptor Histamine Receptor Dopamine Receptor Ras KRas GPR |
|---|---|
| Other CXCR Inhibitors | AZD5069 SB 225002 WZ811 Reparixin (Repertaxin) LIT-927 Navarixin (SCH-527123) UNBS5162 LY2510924 AMD3465 hexahydrobromide AMG 487 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| CHOK1 cells | Function assay | Displacement of [125I]SDF1alpha from CXCR4 expressed in CHOK1 cells, IC50=0.81 nM | 17715128 | |||
| GHOST CXCR4 cell line | Function assay | Inhibitory concentration of compound against HIV-1 LAI strain in GHOST CXCR4 cell line, IC50=0.95 nM | 14698189 | |||
| HEK293 cells | Function assay | 2 days | Antiviral activity against T20-resistant HIV1 NL4-3 infected in HEK293 cells assessed as inhibition of viral replication after 2 days, IC50=2.3 nM | 19451305 | ||
| PBMC cells | Function assay | Effective concentration of compound against HIV-1 89.6 strain in PBMC cells, EC50=3.8 nM | 14698189 | |||
| human MT4 cells | Function assay | 4 days | Antiviral activity against HIV1 3B infected in human MT4 cells assessed as inhibition of virus replication after 4 days by MTT assay, EC50=4 nM | 20043638 | ||
| human Jurkat cells | Function assay | Antagonist activity at CXCR4 in human Jurkat cells assessed as inhibition of SDF1-induced cell migration, IC50=27.4 nM | 19188071 | |||
| MT-4 cells | Function assay | Effective concentration of compound against HIV-1 IIIB strain in MT-4 cells, EC50=65 nM | 14698189 | |||
| rat IR983F cells | Function assay | Displacement of [125I]CXCL12 from CXCR4 in rat IR983F cells, IC50=108 nM | 19053768 | |||
| CEM-SS cells | Function assay | Effective concentration of compound against HIV-1 LAI strain in CEM-SS cells, EC50=127 nM | 14698189 | |||
| human HL60 cells | Function assay | Displacement of [125I]SDF1alpha from CXCR4 in human HL60 cells, IC50=15.2 μM | 19188071 | |||
| human MOLT4 cells | Function assay | 1000 nM | Inhibition of Mab 12G5 binding to CXCR4 expressed in human MOLT4 cells at 1000 nM by FACS analysis | 19451305 | ||
| human MT2 cells | Function assay | 1 ug/mL | 4 days | Antiviral activity against HIV1 3B infected in human MT2 cells assessed as inhibition of viral p24 antigen production at 1 ug/mL after 4 days by ELISA | 21168336 | |
| human U87 cells | Function assay | 1000 nM | Antagonist activity at CXCR4 in human U87 cells assessed as inhibition of SDF1-induced modulation of cAMP production at 1000 nM by TR-FRET assay | 17958344 | ||
| MT4 | Antiviral assay | Antiviral activity against HIV1 3B assessed as reduction in cytopathic effect in MT4 cells, EC50=0.011μM | 17034122 | |||
| U87 | Function assay | 15 mins | Antagonist activity at human CXCR4 expressed in human U87 cells expressing CD4 assessed as inhibition of CXCL12-induced cAMP production pretreated for 15 mins before forskolin challenge by TR-FRET analysis, IC50=0.695μM | 21105715 | ||
| MT4 | Antiviral assay | Antiviral activity against X4-tropic HIV1 NL4.3 infected in human MT4 cells assessed as protection from virus-induced cytopathogenicity, EC50=0.062μM | 22579418 | |||
| MT4 | Antiviral assay | 5 days | Antiviral activity against HIV1 infected in MT4 cells expressing CXCR4 assessed as inhibition of virus-induced cytopathic effect after 5 days, EC50=0.002μM | 22909088 | ||
| CHO | Function assay | Binding affinity to human recombinant CXCR4 expressed in CHO cells, Kb=0.077μM | 22909088 | |||
| CHO | Function assay | Antagonist activity at human recombinant CXCR4 expressed in CHO cells assessed as inhibition of SDF1a-induced electrical impedance by dielectric spectroscopic analysis, IC50=0.26μM | 22909088 | |||
| CD44null | Function assay | 7 days | Effect on human GBM2 cell differentiation assessed as increase in CD44null cells after 7 days by flow cytometric analysis | 22909088 | ||
| CD44null | Function assay | 7 days | Effect on human GBM1 cell differentiation assessed as increase in CD44null cells after 7 days by flow cytometric analysis | 22909088 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
Water : 100 mg/mL
DMSO
: Insoluble
Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 794.47 | Formula | C28H54N8.8HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 155148-31-5 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | JM 3100 8HCl,Plerixafor Octahydrochloride,AMD3100 octahydrochloride,SID791 octahydrochloride | Smiles | C1CNCCNCCCN(CCNC1)CC2=CC=C(C=C2)CN3CCCNCCNCCCNCC3.Cl.Cl.Cl.Cl.Cl.Cl.Cl.Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
CXCL12
(Cell-free assay) 5.7 nM
CXCR4
(Cell-free assay) 44 nM
|
|---|---|
| In vitro |
Plerixafor inhibits CXCL12-mediated chemotaxis with a potency lightly better than its affinity for CXCR4. Plerixafor also antagonizes SDF-1/CXCL12 ligand binding with an IC50 of 651 nM. Plerixafor inhibits SDF-1 mediated GTP-binding, SDF-1 mediated calcium flux and SDF-1 stimulated chemotaxis with IC50 of 27 nM, 572 nM and 51 nM, respectively. Plerixafor does not inhibit calcium flux against cells expressing CXCR3, CCR1, CCR2b, CCR4, CCR5 or CCR7 when stimulated with their cognate ligands, nor does Plerixafor inhibit receptor binding of LTB4. Plerixafor does not, on its own, induce a calcium flux in the CCRF–CEM cells, which express multiple GPCRs including CXCR4, CCR4 and CCR7. |
| In vivo |
A single topical application of Plerixafor promotes wound healing in diabetic mice by increasing cytokine production, mobilizing bone marrow EPCs, and enhancing the activity of fibroblasts and monocytes/macrophages, thereby increasing both angiogenesis and vasculogenesis. Cohorts of mice are administered with PBS, IGF1, PDGF, SCF, or VEGF for five consecutive days and Plerixafor on the 5th day. The number and size of the colonies are highest in IGF1 plus Plerixafor injected mice compared to PDGF, SCF and VEGF treated groups, in combination with Plerixafor. |
References |
|
Applications
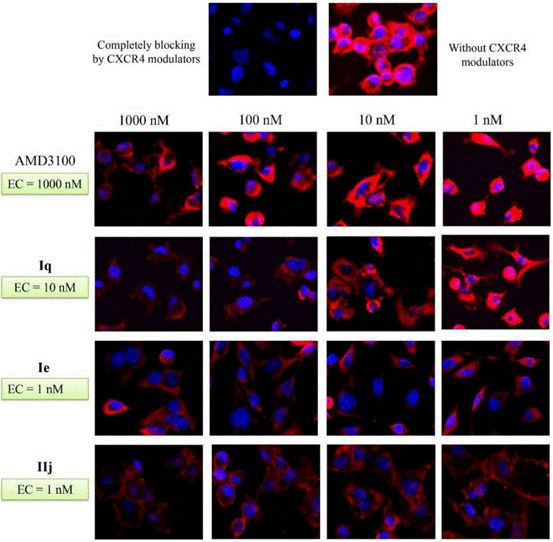
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Immunofluorescence | CXCR4 β-arrestin2 |
|
28521261 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05421416 | Not yet recruiting | Stem Cell Transplant Complications |
AHS Cancer Control Alberta |
April 1 2024 | Phase 2 |
| NCT05343572 | Recruiting | Asherman Syndrome|Atrophic Endometrium|Recurrent Implantation Failure |
Hugh Taylor|Yale University |
November 1 2023 | Early Phase 1 |
| NCT05844527 | Recruiting | Wound of Skin|Abdominal Wound |
MedRegen LLC |
November 20 2023 | Phase 2 |
| NCT05411575 | Withdrawn | COVID-19 Acute Respiratory Distress Syndrome|COVID-19 |
4Living Biotech|4P-Pharma |
July 19 2022 | Phase 2 |
| NCT05445128 | Terminated | Sickle Cell Disease |
Ensoma|bluebird bio |
June 24 2022 | Phase 2 |
| NCT05835726 | Recruiting | Multiple Myeloma|Daratumumab|Autologous Stem Cell Transplantation|Leukapheresis |
Fondazione Policlinico Universitario Agostino Gemelli IRCCS |
January 1 2022 | -- |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































