research use only
Pefloxacin Mesylate Dihydrate Topoisomerase inhibitor
Cat.No.S4119
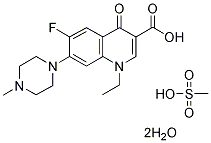
Chemical Structure
Molecular Weight: 465.49
Quality Control
| Related Targets | HDAC PARP ATM/ATR DNA-PK WRN DNA/RNA Synthesis PPAR Sirtuin Casein Kinase eIF |
|---|---|
| Other Topoisomerase Inhibitors | Camptothecin (CPT) Betulinic acid (S)-10-Hydroxycamptothecin Beta-Lapachone Amonafide Ellagic acid Voreloxin (SNS-595) hydrochloride Cu(II)-Elesclomol Hydroxy Camptothecine Rubitecan |
Solubility
|
In vitro |
Water : 67 mg/mL
DMSO
: 9 mg/mL
(19.33 mM)
Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 465.49 | Formula | C17H20FN3O3.CH4O3S.2H2O |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 149676-40-4 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | 1589 RB,Pefloxacinium mesylate dihydrate | Smiles | CCN1C=C(C(=O)C2=CC(=C(C=C21)N3CCN(CC3)C)F)C(=O)O.CS(=O)(=O)O.O.O | ||
Mechanism of Action
| Targets/IC50/Ki |
Topo II
|
|---|---|
| In vitro |
Pefloxacin like that of other quinolones mainly targets bacterial DNA gyrase (topoisomerase II) which is an essential bacterial enzyme with a variety of activities. Pefloxacin is a strongly bactericidal in a complex manner; the bactericidal effect has been described as 'biphasic' with a single rapidly killing effect followed by a paradoxical reduced killing phase in the presence of increased concentration; in bacterial cells incubated with Pefloxacin, non-replicative DNA synthesis (DNA repair), termed SOS response, is induced and SOS response prevents replication, blocking cell division and producing filarnentation. Thus the end result of the SOS response is harmful for the bacteria with morphological and biochemical disturbances. Pefloxacin is highly active against the Enterobacteriaceae (Citrobacter, Enterobacter, Klebsiella, Morganella, Proteus); the minimal inhibitory concentrations (MICs) of Pefloxacin for all genus and species of this family of bacteria ranges usually between 0.03 and 4 or 8 mg/L; against Salmonella, Shigella and Yersinia the MIC50/MIC90 are lower: 0.12/1.0, 0.06/0.06, 0.12/0.25 mg/L, respectively. The MICs of Pefloxacin for Gram- negative aerobic bacilli, such as Pseudomonas aeruginosa, Acinetobacter spp., Alcaligenes, Pseudomonas spp. , ranges from 1 to 4 mg/L. Gram-positive cocci Staphylococci including S. aureus and coagulase-negative staphylococci even resistant to other antibiotics are susceptible or moderately susceptible to Pefloxacin, with MICs in the range 0.125-0.5 mg/L. The MICs of Pefloxacin for Legionella pneumophila are moderately low (MIC50/MIC90:1.0/1.0mg/L); for Eikenella corrodens, Vibrio cholerae, the MICs are very low, whereas Helicobacter pylori, Listeria monocytogenes and Nocardia astero'ides are resistant to Pefloxacin, with MIC50 of 8 mg/L or more. Mycobacteria are moderately or not susceptible to Pefloxacin and most anaerobes are resistant to Pefloxacin. Mycoplasma spp and Chlamydiae are poorly susceptible to Pefloxacin (MIC50/MIC90: 2/8 mg/L) whereas Rickettsia conori and Coxiella burneti are susceptible at therapeutically achievable concentrations. |
References |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































