research use only
Beta-Lapachone Topoisomerase inhibitor
Cat.No.S7261
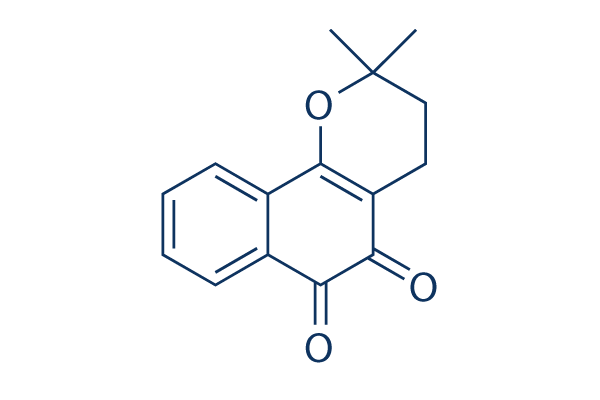
Chemical Structure
Molecular Weight: 242.27
Quality Control
| Related Targets | HDAC PARP ATM/ATR DNA-PK WRN DNA/RNA Synthesis PPAR Sirtuin Casein Kinase eIF |
|---|---|
| Other Topoisomerase Inhibitors | Camptothecin (CPT) Betulinic acid (S)-10-Hydroxycamptothecin Amonafide Ellagic acid Voreloxin (SNS-595) hydrochloride Cu(II)-Elesclomol Hydroxy Camptothecine Rubitecan Genz-644282 |
Solubility
|
In vitro |
DMSO
: 33 mg/mL
(136.21 mM)
Ethanol : 16 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 242.27 | Formula | C15H14O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 4707-32-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | β-Lapachone, ARQ-501 | Smiles | CC1(CCC2=C(O1)C3=CC=CC=C3C(=O)C2=O)C | ||
Mechanism of Action
| Targets/IC50/Ki |
Topo I
IDO1
0.44 μM
|
|---|---|
| In vitro |
Beta-Lapachone inhibits DNA relaxation induced by DNA topoisomerase I in a dose-dependent manner. Treatment of this compound (100 nM or greater) results in >95% inhibition of Topo I DNA unwinding activity compared to the DMSO control. This chemical (1-5 μM) causes a block in G0/G1 of the cell cycle and induces apoptosis by locking Topo I onto DNA and blocking replication fork movement in HL-60 and three human prostate cancer (DU-145, PC-3, and LNCaP) cells. It facilitates the migration of mouse 3T3 fibroblasts and human endothelial EAhy926 cells through different MAPK signaling pathways, and thus accelerates scrape-wound healing in vitro. In addition, this compound inhibits purified recombinant IDO1 activity through uncompetitive inhibition with IC50 of 0.44 μM, and it also exhibits superior retention of intracellular IDO1 inhibitory activity with an IC50 of 1.0 μM, partially dependent on biotransformation by NQO1. This chemical induces programmed necrosis of NQO1+ cancer cells by NQO1-dependent reactive oxygen species (ROS) formation and PARP1 hyperactivation. [5]
|
| Kinase Assay |
Topoisomerase I Catalytic Actioity Assay
|
|
Topoisomerase I Catalytic Activity Assay: The enzymatic activity is analyzed by the DNA unwinding assay. DNA topoisomerase I, from TopoGEN (1 unit, which is defined as the amount of enzyme that converts 0.5 μg of superhelical DNA to the relaxed state in 30 minutes at 37 °C), is incubated with 0.5 μg of 6x174 RF DNA, in the presence or absence of Beta-Lapachone, in 20 μL of relaxation buffer (50 mM Tris (pH 7.5), 50 mM KCI, 10 mM MgCl2, 0.5 mM dithiothreitol, 0.5 mM EDTA, 30 μg/mL bovine serum albumin) for 30 minutes at 37 °C. Reactions are stopped by adding 1% SDS and proteinase K (50 μg/mL). After an additional 1-hour incubation at 37 °C, the products are separated by electrophoresis in 1% agarose gel in TAE buffer (0.04 M tris acetate, 0.001 M EDTA). The gel is stained with ethidium bromide after electrophoresis. The photographic negative is scanned with an NIH image analysis system.
|
|
| In vivo |
Beta-lapachone treatment (50 mg/kg) leads to potent inhibition of in vivo tumor growth in a xenograft mouse model of human ovarian cancer, and the combination of this compound and taxol produces a synergistic induction of apoptosis. In normal and diabetic (db/db) mice, treatment of this chemical results in a faster healing process than vehicle only.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT00524524 | Completed | Advanced Solid Tumors |
ArQule Inc. a subsidiary of Merck Sharp & Dohme LLC a subsidiary of Merck & Co. Inc. (Rahway NJ USA) |
August 2007 | Phase 1 |
| NCT00622063 | Completed | Cancer |
ArQule Inc. a subsidiary of Merck Sharp & Dohme LLC a subsidiary of Merck & Co. Inc. (Rahway NJ USA) |
December 2006 | Phase 1|Phase 2 |
| NCT00358930 | Completed | Head and Neck Neoplasms|Carcinoma Squamous Cell |
ArQule Inc. a subsidiary of Merck Sharp & Dohme LLC a subsidiary of Merck & Co. Inc. (Rahway NJ USA) |
July 2006 | Phase 2 |
| NCT00102700 | Completed | Pancreatic Cancer|Adenocarcinoma |
ArQule Inc. a subsidiary of Merck Sharp & Dohme LLC a subsidiary of Merck & Co. Inc. (Rahway NJ USA) |
January 2005 | Phase 2 |
| NCT00099190 | Completed | Carcinoma |
ArQule Inc. a subsidiary of Merck Sharp & Dohme LLC a subsidiary of Merck & Co. Inc. (Rahway NJ USA) |
December 2004 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































