research use only
LGX818 (Encorafenib) BRAF inhibitor
Cat.No.S7108
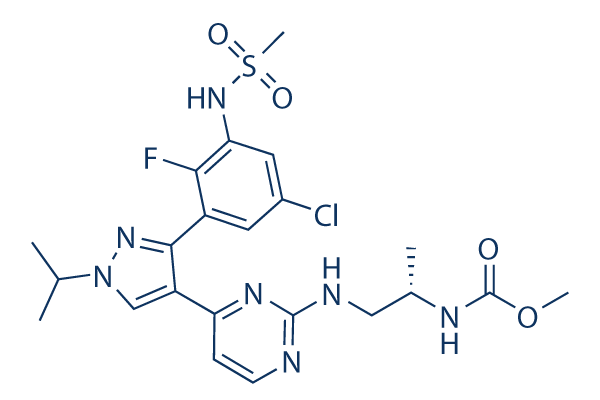
Chemical Structure
Molecular Weight: 540.01
Quality Control
| Related Targets | ERK p38 MAPK JNK MEK Ras KRas S6 Kinase MAP4K TAK1 Mixed Lineage Kinase |
|---|---|
| Other Raf Inhibitors | LY3009120 Exarafenib (KIN-2787) GDC-0879 Avutometinib (Ro5126766, CH5126766) PLX-4720 AZ 628 SB590885 TAK-632 GW5074 RAF265 (CHIR-265) |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| A375 | Function assay | 2 days | Inhibition of B-Raf V600E mutant in human A375 cells harboring B-Raf V600E mutant assessed as reduction in cell proliferation incubated for 2 days by Bright-Glo luminescence assay, IC50 = 0.002 μM. | ChEMBL | ||
| A549 | Function assay | 30 mins | Inhibition of IL1-beta-induced p38alpha phosphorylation in human A549 cells pre-incubated for 30 mins before IL1-beta stimulation and measured after 7 to 8 hrs post IL1-beta stimulation by Bright-Glo luciferase reporter gene assay, IC50 = 2.2 μM. | ChEMBL | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(185.18 mM)
Ethanol : 7 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 540.01 | Formula | C22 H27 Cl F N7 O4 S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1269440-17-6 | -- | Storage of Stock Solutions |
|
|
| Synonyms | LGX818 | Smiles | CC(C)N1C=C(C(=N1)C2=C(C(=CC(=C2)Cl)NS(=O)(=O)C)F)C3=NC(=NC=C3)NCC(C)NC(=O)OC | ||
Mechanism of Action
| Features |
Orally bioavailable RAF-selective inhibitor.
|
|---|---|
| Targets/IC50/Ki |
B-Raf (V600E)
|
| In vitro |
In the A375 (BRAFV600E) human melanoma cell line, Encorafenib (LGX818) suppresses phospho-ERK (EC50 = 3 nM) leading to potent inhibition of proliferation (EC50 = 4 nM). No significant activity is observed against a panel of 100 kinases (IC50 > 900 nM), and it does not inhibit proliferation of > 400 cell lines expressing wild-type BRAF. Contributing to the high potency of this compound is the extremely slow off-rate from BRAFV600E, which is not observed with other RAF inhibitors. In biochemical assays, the dissociation half-life is >24 hours, which translated into sustained target inhibition in cells following drug wash-out. |
| In vivo |
Encorafenib (LGX818) is a potent and selective RAF kinase inhibitor with unique biochemical properties that contribute to an excellent pharmacological profile. Treatment with this compound at oral doses as low as 6 mg/kg resulted in strong (75%) and sustained (>24 hours) decrease in phospho-MEK, even following clearance of drug from circulation in single dose PK/PD studies in human melanoma xenograft models (BRAFV600E). It induces tumor regression in multiple BRAF mutant human tumor xenograft models grown in immune compromised mice and rats at doses as low as 1 mg/kg. Consistent with the in vitro data, it is inactive against BRAF wild-type tumors at doses up to 300 mg/kg bid, with good tolerability and linear increase in exposure. Efficacy is also achieved in a more disease-relevant spontaneous metastatic melanoma and a model of melanoma brain metastasis. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | p-ERK / ERK / p-MEK / MEK / p-p90RSK / p90RSK BIM / Mcl-1 / Bcl2 / Bcl-xl / NOXA / MITF / c-myc / PARP / Cleaved caspase Cyclin D1 / CDC6 / CDK2 p21CIP1 / p27KIP1 / pRb p-mTOR / pp70S6K |
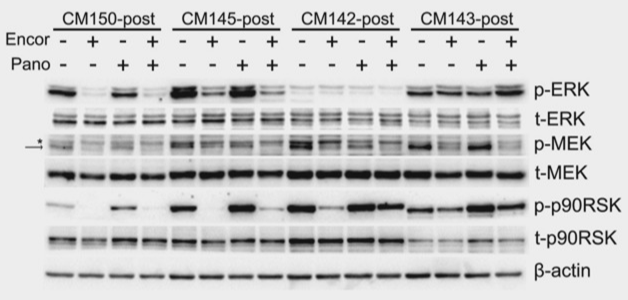
|
29210065 |
| Growth inhibition assay | Cell viability |
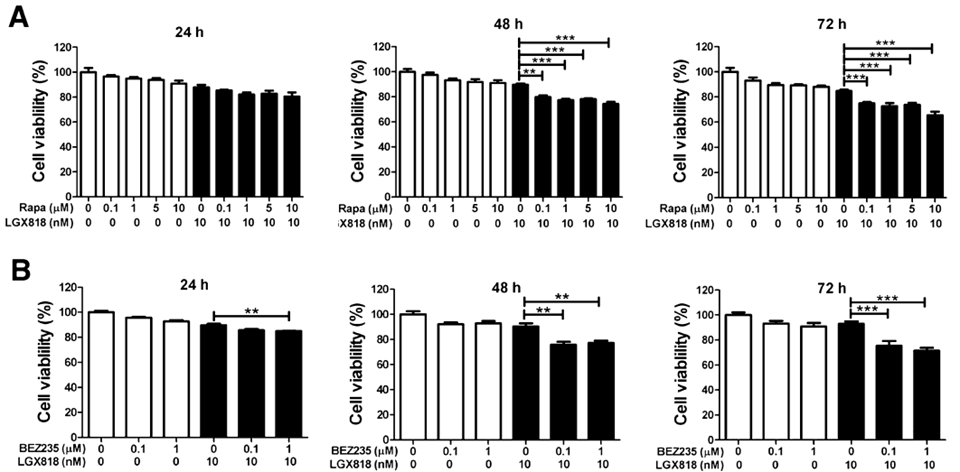
|
26586345 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02834364 | Completed | Relapsed or Refractory Multiple Myeloma|Patients With BRAFV600 E or BRAFV600K Mutation |
University of Heidelberg Medical Center|Array BioPharma|German Cancer Research Center|Coordinating Centre for Clinical Trials Heidelberg|University Hospital Heidelberg |
June 2016 | Phase 2 |
| NCT02278133 | Completed | Metastatic Colorectal Cancer |
Array BioPharma |
December 2014 | Phase 1|Phase 2 |
| NCT01909453 | Active not recruiting | Melanoma |
Pfizer |
September 16 2013 | Phase 3 |
| NCT01719380 | Completed | Colorectal Cancer |
Pfizer |
November 23 2012 | Phase 2 |
| NCT01436656 | Completed | Melanoma and Metastatic Colorectal Cancer |
Pfizer |
September 5 2011 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































