research use only
Tovorafenib (MLN2480) Raf Kinase Inhibitor
Cat.No.S7121
Chemical Structure
Molecular Weight: 506.29
Quality Control
| Related Targets | ERK p38 MAPK JNK MEK Ras KRas S6 Kinase MAP4K TAK1 Mixed Lineage Kinase |
|---|---|
| Other Raf Inhibitors | LY3009120 Exarafenib (KIN-2787) GDC-0879 Avutometinib (Ro5126766, CH5126766) PLX-4720 AZ 628 SB590885 TAK-632 GW5074 RAF265 (CHIR-265) |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(197.51 mM)
Ethanol : 100 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 506.29 | Formula | C17H12Cl2F3N7O2S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1096708-71-2 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | BIIB-024, TAK580, AMG-2112819, BSK1369, DAY-101 | Smiles | CC(C1=NC=C(S1)C(=O)NC2=NC=C(C(=C2)C(F)(F)F)Cl)NC(=O)C3=C(C(=NC=N3)N)Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
Raf
|
|---|---|
| In vitro |
Tovorafenib (MLN2480) inhibits MAPK pathway signaling in BRAF mutant and some RAS mutant preclinical cancer models at concentrations that are tolerated in vivo. It is found to activate phosphorylated MEK at very low concentrations, but inhibits this same activity at higher concentrations. The inhibitory effects of this compound are found to vary across models and genetic contexts. In vitro analysis of the drug combination of MLN2480 and TAK-733 (an investigational allosteric MEK kinase inhibitor) in cell proliferation assays demonstrates synergistic activity. In addition, western blot analysis demonstrates the effect of it in reversing feedback activation of MEK in response to TAK-733, leading to more concerted MAPK pathway inhibition. It only modestly inhibits PRAK. |
| In vivo |
In vivo, Tovorafenib (MLN2480) shows antitumor activity in melanoma, colon, lung, and pancreatic cancer xenograft models. This compound (37.5 mg/kg) is well tolerated in a tumor xenograft model. The combination of it (12.5 mg) and TAK-733 (1 mg/kg) is effective in an SK-MEL-30 xenograft model, but monotherapy with either compound produces negligible effects. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | p-ERK / ERK |
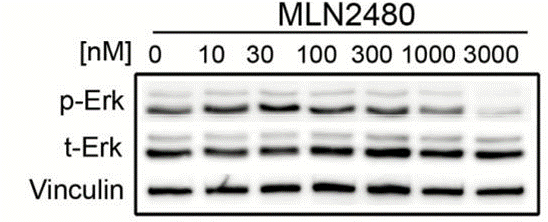
|
28082416 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06381570 | Recruiting | Low-grade Glioma |
Daniel Morgenstern|The Hospital for Sick Children |
March 21 2024 | Early Phase 1 |
| NCT05566795 | Recruiting | Low-grade Glioma |
Day One Biopharmaceuticals Inc.|SIOPe Brain Tumor Group LOGGIC Consortium |
February 27 2023 | Phase 3 |
| NCT04985604 | Recruiting | Melanoma|Solid Tumor|CRAF Gene Amplification|RAF1 Gene Amplification|BRAF Gene Fusion|BRAF Fusion|CRAF Gene Fusion|CRAF Fusion|RAF1 Gene Fusion|RAF1 Fusion|Thyroid Cancer Papillary|Spitzoid Melanoma|Pilocytic Astrocytoma|Pilocytic Astrocytoma Adult|Non Small Cell Lung Cancer|Non-Small Cell Adenocarcinoma|Colorectal Cancer|Pancreatic Acinar Carcinoma|Spitzoid Malignant Melanoma|Bladder Cancer|Bladder Urothelial Carcinoma|MAP Kinase Family Gene Mutation|RAS Mutation|RAF Mutation|MEK Mutation |
Day One Biopharmaceuticals Inc. |
July 15 2021 | Phase 1|Phase 2 |
| NCT04775485 | Recruiting | Low-grade Glioma|Advanced Solid Tumor |
Day One Biopharmaceuticals Inc.|Pacific Pediatric Neuro-Oncology Consortium |
April 22 2021 | Phase 2 |
| NCT03429803 | Active not recruiting | Low-grade Glioma |
Karen D. Wright MD|PLGA Fund at Pediatric Brain Tumor Foundation|National Cancer Institute (NCI)|Pacific Pediatric Neuro-Oncology Consortium|Team Jack Foundation|Day One Biopharmaceuticals Inc.|Dana-Farber Cancer Institute |
February 27 2018 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































