|
How to Cite 1. For In-Text Citation (Materials & Methods): 2. For Key Resources Table: |
||
|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. We strive to reply to |
Technical Data
| Formula | C20H24FN5O3 |
||||||
| Molecular Weight | 401.43 | CAS No. | 873697-71-3 | ||||
| Solubility (25°C)* | In vitro | DMSO | 80 mg/mL (199.28 mM) | ||||
| Ethanol | 14 mg/mL (34.87 mM) | ||||||
| Water | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Omecamtiv mecarbil (CK-1827452) is a specific cardiac myosin activator and a clinical drug for left ventricular systolic heart failure, currently in Phase 2. | |
|---|---|---|
| Targets |
|
|
| In vitro | In vitro, Omecamtiv mecarbil (CK-1827452) selectively activates cardiac myosin by increasing the myosin ATPase rate. In isolated cardiac myocytes, it results in increase of myocyte contractility and overcomes of the myosin inhibitor BDM without increasing the calcium transient or inhibiting the PDE pathway. | |
| In vivo | Omecamtiv mecarbil (CK-1827452) significantly increases fractional shortening starting at 0.4 mM plasma concentrations in SD rats, sham animals and in rats with heart failure. In conscious dogs with myocardial infarction (MI-sHF), it leads to a significant increase in wall thickening (25%), stroke volume (44%), cardiac output (22%) and left ventricular (LV) systolic ejection time (26%). In addition, this compound also results in the decreases of some hemodynamic parameters including heart rate, mean left atrial pressure, and LV end-diastolic pressure. In conscious dogs with left ventricular hypertrophy (LVH-sHF), it leads to similar and not statistically different effects on hemodynamic parameters. |
Protocol (from reference)
| Animal Study:[1] |
|
|---|
References
|
Customer Product Validation
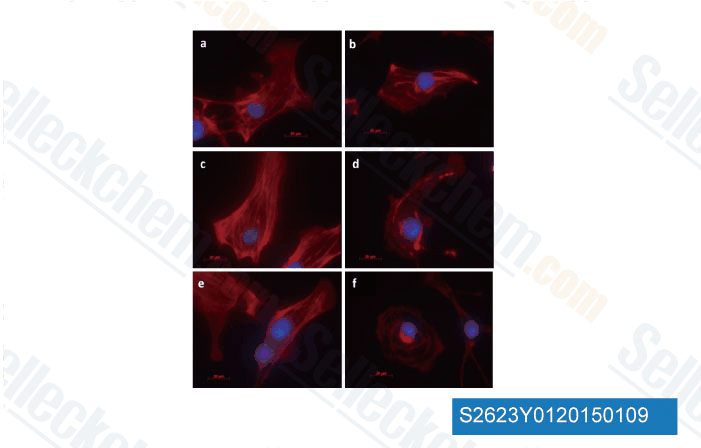
-
Data from [ University of Illinois at Chicago , 2014 ]
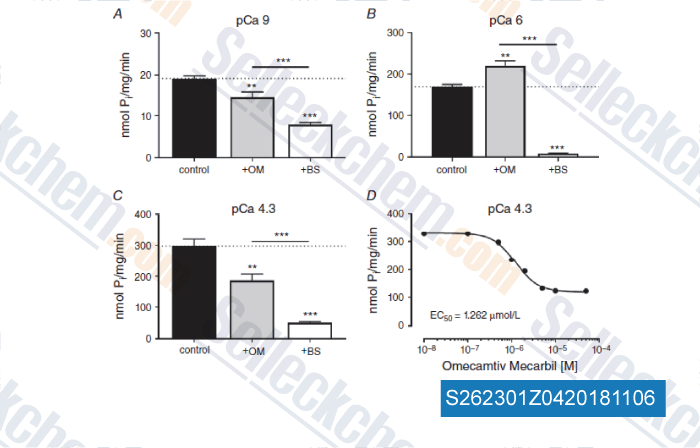
-
Data from [ , , J Physiol, 2018, 596(1):31-46 ]
Selleck's Omecamtiv mecarbil (CK-1827452) Has Been Cited by 35 Publications
| Single-cell polygenic risk scores dissect cellular and molecular heterogeneity of complex human diseases [ Nat Biotechnol, 2025, 10.1038/s41587-025-02725-6] | PubMed: 40715455 |
| Osimertinib induces reversible cardiac dysfunction through the GATA4-MYLK3-MYL2 axis [ Eur Heart J, 2025, ehaf813] | PubMed: 41330421 |
| Characterization of NEB pathogenic variants in patients reveals novel nemaline myopathy disease mechanisms and omecamtiv mecarbil force effects [ Acta Neuropathol, 2024, 147(1):72] | PubMed: 38634969 |
| Targeting ErbB and tankyrase1/2 prevent the emergence of drug-tolerant persister cells in ALK-positive lung cancer [ NPJ Precis Oncol, 2024, 8(1):264] | PubMed: 39551860 |
| Deconvolution of polygenic risk score in single cells unravels cellular and molecular heterogeneity of complex human diseases [ bioRxiv, 2024, 2024.05.14.594252] | PubMed: 38798507 |
| Clinically Relevant Models of Cardiac Function using Human Stem Cell-derived Cardiomyocytes [ , 2023, ] | PubMed: none |
| The force of the myosin motor sets cooperativity in thin filament activation of skeletal muscles [ Commun Biol, 2022, 5(1):1266] | PubMed: 36400920 |
| Effects of omecamtiv mecarbil and mavacamten in isolated human atrium [ Naunyn Schmiedebergs Arch Pharmacol, 2022, 10.1007/s00210-022-02333-0] | PubMed: 36399186 |
| Myosin with hypertrophic cardiac mutation R712L has a decreased working stroke which is rescued by omecamtiv mecarbil [ Elife, 2021, 10e63691] | PubMed: 33605878 |
| Mechanistic analysis of actin-binding compounds that affect the kinetics of cardiac myosin-actin interaction [ J Biol Chem, 2021, S0021-9258(21)00245-3] | PubMed: 33639160 |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
