research use only
Irinotecan Hydrochloride Trihydrate Topoisomerase I Inhibitor
Cat.No.S2217
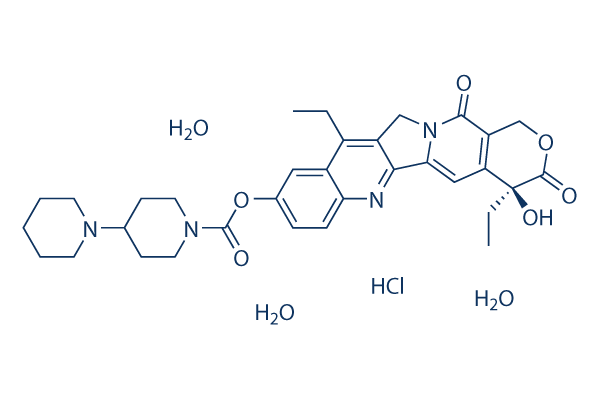
Chemical Structure
Molecular Weight: 677.18
Quality Control
| Related Targets | HDAC PARP ATM/ATR DNA-PK WRN DNA/RNA Synthesis PPAR Sirtuin Casein Kinase eIF |
|---|---|
| Other Topoisomerase Inhibitors | Camptothecin (CPT) Betulinic acid (S)-10-Hydroxycamptothecin Beta-Lapachone Amonafide Ellagic acid Voreloxin (SNS-595) hydrochloride Cu(II)-Elesclomol Hydroxy Camptothecine Rubitecan |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| RPMI8402 | Cytotoxicity assay | 4 days | Cytotoxic activity against human lymphoblast tumor cell line RPMI8402 after 4 days of treatment. IC50=0.57 μM | 12747798 | ||
| Hep3B | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Hep3B cells after 72 hrs by XTT assay, GI50 = 4.73 μM. | 19796956 | ||
| HepG2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HepG2 cells after 72 hrs by XTT assay, GI50 = 5.94 μM. | 19796956 | ||
| NCI60 | Growth inhibition assay | Growth inhibition of human NCI60 cells by SBR assay, GI50 = 14.1254 μM. | 21035334 | |||
| Hep3B | Growth inhibition assay | 72 hrs | Growth inhibition of human Hep3B cells after 72 hrs by XTT assay, GI50 = 4.73 μM. | 22079254 | ||
| HepG2 | Growth inhibition assay | 72 hrs | Growth inhibition of human HepG2 cells after 72 hrs by XTT assay, GI50 = 5.94 μM. | 22079254 | ||
| MDA-MB-231 | Growth inhibition assay | 72 hrs | Growth inhibition of human MDA-MB-231 cells after 72 hrs by XTT assay, GI50 = 9.17 μM. | 22079254 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells incubated for 72 hrs by MTT assay, GI50 = 5 μM. | 22867019 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells incubated for 72 hrs by MTT assay, GI50 = 7.1 μM. | 22867019 | ||
| MDA-MB-435 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-435 cells incubated for 72 hrs by MTT assay, GI50 = 12.2 μM. | 22867019 | ||
| K562 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human K562 cells after 72 hrs, GI50 = 1.9 μM. | 25420175 | ||
| A549 | Antitumor assay | 4 hrs | Antitumor activity against human A549 cells after 4 hrs by MTT assay, IC50 = 6.528 μM. | 18207748 | ||
| LOVO | Antitumor assay | 4 hrs | Antitumor activity against human LOVO cells after 4 hrs by MTT assay, IC50 = 9.015 μM. | 18207748 | ||
| MCF7 | Antitumor assay | 4 hrs | Antitumor activity against human MCF7 cells after 4 hrs by MTT assay, IC50 = 17.403 μM. | 18207748 | ||
| LS174T | Cytotoxicity assay | 96 hrs | Cytotoxicity against human LS174T cells after 96 hrs by MTT assay, IC50 = 1.16 μM. | 18513976 | ||
| KB3-1 | Cytotoxicity assay | Cytotoxicity against human KB3-1 cells by MTT method, IC50 = 0.68 μM. | 18771930 | |||
| KBH5.0 | Cytotoxicity assay | Cytotoxicity against human KBH5.0 cells by MTT method, IC50 = 7.4 μM. | 18771930 | |||
| KBV1 | Cytotoxicity assay | Cytotoxicity against human KBV1 cells by MTT method, IC50 = 40 μM. | 18771930 | |||
| MDA-MB-435 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-435 cells after 72 hrs by MTT assay, IC50 = 1.14 μM. | 20371183 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by MTT assay, IC50 = 4.61 μM. | 20371183 | ||
| LoVo | Cytotoxicity assay | 72 hrs | Cytotoxicity against human LoVo cells after 72 hrs by MTT assay, IC50 = 4.99 μM. | 20371183 | ||
| A549 | Cytotoxicity assay | 3 days | Cytotoxicity against human A549 cells after 3 days by MTT assay, IC50 = 6.4 μM. | 20942490 | ||
| LoVo | Cytotoxicity assay | 3 days | Cytotoxicity against human LoVo cells after 3 days by MTT assay, IC50 = 8.8 μM. | 20942490 | ||
| MDA-MB-435 | Cytotoxicity assay | 3 days | Cytotoxicity against human MDA-MB-435 cells after 3 days by MTT assay, IC50 = 17 μM. | 20942490 | ||
| A375 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A375 cells after 72 hrs by alamar blue assay, IC50 = 0.004 μM. | 21341674 | ||
| PC3 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human PC3 cells after 72 hrs by alamar blue assay, IC50 = 0.004 μM. | 21341674 | ||
| LNCAP | Cytotoxicity assay | 72 hrs | Cytotoxicity against human LNCAP cells after 72 hrs by alamar blue assay, IC50 = 0.009 μM. | 21341674 | ||
| MESSA | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MESSA cells after 72 hrs by alamar blue assay, IC50 = 0.01 μM. | 21341674 | ||
| H460 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human H460 cells after 72 hrs by alamar blue assay, IC50 = 0.015 μM. | 21341674 | ||
| H69 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human H69 cells after 72 hrs by alamar blue assay, IC50 = 0.022 μM. | 21341674 | ||
| MES-SA/Dx5 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MES-SA/Dx5 cells overexpressing MDR1 after 72 hrs by alamar blue assay, IC50 = 0.022 μM. | 21341674 | ||
| H460 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human H460 cells after 72 hrs by alamar blue assay in presence of 40 mg/ml HSA, IC50 = 0.027 μM. | 21341674 | ||
| IGROV1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human IGROV1 cells after 72 hrs by alamar blue assay, IC50 = 0.03 μM. | 21341674 | ||
| SK-MEL-2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human SK-MEL-2 cells after 72 hrs by alamar blue assay, IC50 = 0.1 μM. | 21341674 | ||
| MALME-3M | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MALME-3M cells after 72 hrs by alamar blue assay, IC50 = 0.2 μM. | 21341674 | ||
| DU145 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human DU145 cells after 72 hrs by alamar blue assay, IC50 = 0.2 μM. | 21341674 | ||
| HT-29 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HT-29 cells after 72 hrs by alamar blue assay, IC50 = 0.22 μM. | 21341674 | ||
| H69AR | Cytotoxicity assay | 72 hrs | Cytotoxicity against human H69AR cells overexpressing MDR1 after 72 hrs by alamar blue assay, IC50 = 0.25 μM. | 21341674 | ||
| DU145 | Cytotoxicity assay | Cytotoxicity against human DU145 cells by SRB assay, IC50 = 9.3 μM. | 23102893 | |||
| A549 | Cytotoxicity assay | Cytotoxicity against human A549 cells by SRB assay, IC50 = 9.48 μM. | 23102893 | |||
| KB | Cytotoxicity assay | Cytotoxicity against human KB cells by SRB assay, IC50 = 9.83 μM. | 23102893 | |||
| HEK293 | Inhibition of human | 1.5 mins | Inhibition of human MATE1-mediated [14]-metformin uptake expressed in HEK293 cells after 1.5 mins by scintillation counting analysis, IC50 = 2.1 μM. | 23241029 | ||
| HEK293 | Inhibition of human | 3 mins | Inhibition of human OCT2-mediated ASP+ uptake expressed in HEK293 cells after 3 mins by fluorescence assay, IC50 = 2.7 μM. | 23241029 | ||
| MDCK2 | Inhibition of human | 5 mins | Inhibition of human MATE1-mediated [14]-metformin uptake expressed in polarized MDCK2 cells after 5 mins by liquid scintillation counting analysis, IC50 = 4.3 μM. | 23241029 | ||
| HEK293 | Inhibition of human | up to 500 uM | 1.5 mins | Inhibition of human MATE2K-mediated ASP+ uptake expressed in HEK293 cells up to 500 uM after 1.5 mins by fluorescence assay, IC50 = 7.9 μM. | 23241029 | |
| HEK293 | Inhibition of human | 1.5 mins | Inhibition of human MATE1-mediated ASP+ uptake expressed in HEK293 cells after 1.5 mins by fluorescence assay, IC50 = 7.9 μM. | 23241029 | ||
| HEK293 | Inhibition of human | 3 mins | Inhibition of human OCT1-mediated ASP+ uptake expressed in HEK293 cells after 3 mins by fluorescence assay, IC50 = 20.8 μM. | 23241029 | ||
| H3347 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human H3347 cells assessed as growth inhibition after 72 hrs by Alamar Blue assay, IC50 = 7.53 μM. | 24583355 | ||
| DLD1 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human DLD1 cells assessed as growth inhibition after 72 hrs by Alamar Blue assay, IC50 = 25.1 μM. | 24583355 | ||
| DU145 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human DU145 cells after 72 hrs by SRB assay, IC50 = 9.3 μM. | 25003995 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by SRB assay, IC50 = 9.5 μM. | 25003995 | ||
| KB | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB cells after 72 hrs by SRB assay, IC50 = 9.8 μM. | 25003995 | ||
| MDA-MB-435 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-435 cells after 72 hrs by MTT assay, IC50 = 6.3 μM. | 26226379 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by MTT assay, IC50 = 18.6 μM. | 26226379 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by MTT assay, IC50 = 20.1 μM. | 26226379 | ||
| HCT116 | Antiproliferative assay | 24 to 72 hrs | Antiproliferative activity against human HCT116 cells after 24 to 72 hrs by SRB assay, IC50 = 2 μM. | 26595875 | ||
| RKO | Antiproliferative assay | 24 to 72 hrs | Antiproliferative activity against human RKO cells after 24 to 72 hrs by SRB assay, IC50 = 6.2 μM. | 26595875 | ||
| HCT15 | Antiproliferative assay | 24 to 72 hrs | Antiproliferative activity against human HCT15 cells after 24 to 72 hrs by SRB assay, IC50 = 8.5 μM. | 26595875 | ||
| SW480 | Antiproliferative assay | 24 to 72 hrs | Antiproliferative activity against human SW480 cells after 24 to 72 hrs by SRB assay, IC50 = 12.3 μM. | 26595875 | ||
| PC3 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PC3 cells assessed as cellular DNA content after 72 hrs by CyQUANT NF fluorescence assay, IC50 = 0.8 μM. | 26731300 | ||
| MCF7 | Antiproliferative assay | 96 hrs | Antiproliferative activity against human MCF7 cells assessed as cellular DNA content after 96 hrs by CyQUANT NF fluorescence assay, IC50 = 0.9 μM. | 26731300 | ||
| T84 | Antiproliferative assay | 96 hrs | Antiproliferative activity against human T84 cells assessed as cellular DNA content after 96 hrs by CyQUANT NF fluorescence assay, IC50 = 1.2 μM. | 26731300 | ||
| HT-29 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HT-29 cells assessed as cellular DNA content after 72 hrs by CyQUANT NF fluorescence assay, IC50 = 1.9 μM. | 26731300 | ||
| MCF7 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human MCF7 cells assessed as reduction in cell viability after 24 hrs by MTS assay, IC50 = 0.5 μM. | 26841168 | ||
| A549 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human A549 cells assessed as reduction in cell viability after 24 hrs by MTS assay, IC50 = 0.8 μM. | 26841168 | ||
| HepG2 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human HepG2 cells assessed as reduction in cell viability after 24 hrs by MTS assay, IC50 = 0.9 μM. | 26841168 | ||
| SW480 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human SW480 cells assessed as reduction in cell viability after 24 hrs by MTS assay, IC50 = 1.4 μM. | 26841168 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by sulforhodamine B colorimetric assay, IC50 = 9.48 μM. | 26994847 | ||
| KB | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB cells after 72 hrs by sulforhodamine B colorimetric assay, IC50 = 9.828 μM. | 26994847 | ||
| KB | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB cells after 72 hrs by sulforhodamine B assay, IC50 = 7.99 μM. | 28285912 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by sulforhodamine B assay, IC50 = 8.3 μM. | 28285912 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by sulforhodamine B assay, IC50 = 11.3 μM. | 28285912 | ||
| MDA-MB-231 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-231 cells after 72 hrs by sulforhodamine B assay, IC50 = 15.6 μM. | 28285912 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells after 72 hrs by MTT assay, IC50 = 6.3 μM. | 28351590 | ||
| ZR-7530 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human ZR-7530 cells after 72 hrs by MTT assay, IC50 = 18 μM. | 28351590 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells after 72 hrs by MTT assay, IC50 = 20 μM. | 28351590 | ||
| KB | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB cells after 72 hrs by SRB assay, IC50 = 7.99 μM. | 28789891 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by SRB assay, IC50 = 8.31 μM. | 28789891 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by SRB assay, IC50 = 11.32 μM. | 28789891 | ||
| MDA-MB-231 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-231 cells after 72 hrs by SRB assay, IC50 = 15.56 μM. | 28789891 | ||
| KB | Cytotoxicity assay | 72 hrs | Cytotoxicity against human KB cells after 72 hrs by SRB assay, IC50 = 7.99 μM. | 28927790 | ||
| A549 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human A549 cells after 72 hrs by SRB assay, IC50 = 8.3 μM. | 28927790 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by SRB assay, IC50 = 11.3 μM. | 28927790 | ||
| MDA-MB-231 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA-MB-231 cells after 72 hrs by SRB assay, IC50 = 15.6 μM. | 28927790 | ||
| Maver2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Maver2 cells after 72 hrs by MTT assay, IC50 = 0.74 μM. | 29150335 | ||
| NB4 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human NB4 cells after 72 hrs by MTT assay, IC50 = 1 μM. | 29150335 | ||
| Capan1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human Capan1 cells after 72 hrs by SRB assay, IC50 = 1.5 μM. | 29150335 | ||
| NCI-H460 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human NCI-H460 cells after 72 hrs by SRB assay, IC50 = 2.4 μM. | 29150335 | ||
| DU145 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human DU145 cells after 72 hrs by SRB assay, IC50 = 2.8 μM. | 29150335 | ||
| A2780 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A2780 cells after 72 hrs by SRB assay, IC50 = 3.8 μM. | 29150335 | ||
| HepG2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HepG2 cells after 72 hrs by SRB assay, IC50 = 4 μM. | 29150335 | ||
| A431 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A431 cells after 72 hrs by SRB assay, IC50 = 4.1 μM. | 29150335 | ||
| U2932 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human U2932 cells after 72 hrs by MTT assay, IC50 = 4.4 μM. | 29150335 | ||
| HT-29 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HT-29 cells after 72 hrs by SRB assay, IC50 = 4.8 μM. | 29150335 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells after 72 hrs by SRB assay, IC50 = 5.8 μM. | 29150335 | ||
| MM487 | Antiproliferative assay | 10 uM | 72 hrs | Antiproliferative activity against human MM487 cells at 10 uM after 72 hrs by MTT assay relative to control, IC50 = 9.4 μM. | 29150335 | |
| MM432 | Antiproliferative assay | 10 uM | 72 hrs | Antiproliferative activity against human MM432 cells at 10 uM after 72 hrs by MTT assay relative to control, IC50 = 10 μM. | 29150335 | |
| A2780/DX | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A2780/DX cells after 72 hrs by SRB assay, IC50 = 20.1 μM. | 29150335 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells after 72 hrs by SRB assay, IC50 = 1.63347 μM. | 30115492 | ||
| A549 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A549 cells after 72 hrs by SRB assay, IC50 = 1.88093 μM. | 30115492 | ||
| colon cancer cells | Cytotoxicity assay | Compound was tested in vitro for cytotoxicity against HCT116, human colon cancer cells (taxol-resistant) at a drug concentration producing 50% inhibition of colony formation, ID50 = 0.54 μM. | 11965362 | |||
| SW620 | Antitumor assay | 12.5 mg/kg | Antitumor activity against human SW620 cells xenografted in nude SCID mouse assessed as delay in tumor growth at 12.5 mg/kg, ip | 22111927 | ||
| HT-29 | Antitumor assay | 60 mg/kg | 2 days | Antitumor activity against human HT-29 cells xenografted in nude mouse assessed as tumor growth inhibition at 60 mg/kg, iv tid administered 2 days measured on day 20 | 23360284 | |
| BT-483 | Function assay | 1 uM | 24 hrs | Induction of DNA damage in human BT-483 cells assessed as gamma H2AX phosphorylation at Ser139 at 1 uM after 24 hrs by Western blot analysis | 23419737 | |
| HCT116 | Antitumor assay | 30 mg/kg | 6 days | Antitumor activity against human HCT116 cells xenografted in nude mouse assessed as survival at 30 mg/kg,iv qd administered for 6 days | 24583355 | |
| H3347 | Antitumor assay | 30 mg/kg | 6 days | Antitumor activity against human H3347 cells xenografted in nude mouse assessed as survival at 30 mg/kg,iv qd administered for 6 days | 24583355 | |
| HCT116 | Antitumor assay | 100 mg/kg | Antitumor activity against human HCT116 cells xenografted in nu/nu mouse assessed as tumor regression at 100 mg/kg, iv dosed once every week | 25003995 | ||
| NCI-H526 | Antitumor assay | 30 mg/kg | Antitumor activity against human NCI-H526 cells xenografted in athymic nude mouse at 30 mg/kg, iv qd administered 5 times for every 2 days | 28064078 | ||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for TC32 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for MG 63 (6-TG R) cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for LAN-5 cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for NB-EBc1 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for A673 cells) | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for BT-37 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for MG 63 (6-TG R) cells | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh30 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for OHS-50 cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Orthogonal 3D viability screen for SK-N-SH cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh41 cells | 29435139 | |||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Orthogonal 3D viability screen for TC32 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Orthogonal 3D viability screen for MG 63 (6-TG R) cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh18 cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for NB1643 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SK-N-MC cells | 29435139 | |||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for TC32 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh18 cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Saos-2 cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Orthogonal 3D viability screen for SJ-GBM2 cells | 29435139 | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(147.67 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 677.18 | Formula | C33H38N4O6.HCl.3H2O |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 136572-09-3 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | CPT-11 HCl Trihydrate | Smiles | CCC1=C2CN3C(=CC4=C(C3=O)COC(=O)C4(CC)O)C2=NC5=C1C=C(C=C5)OC(=O)N6CCC(CC6)N7CCCCC7.O.O.O.Cl | ||
Mechanism of Action
| Features |
Irinotecan is a prodrug that is used to treat metastatic colorectal cancer.
|
|---|---|
| Targets/IC50/Ki |
Topo I
|
| In vitro |
Irinotecan is activated to SN-38 by carboxylesterases to become able to interact with its target, topoisomerase I. Irinotecan induces similar amounts of cleavable complexes at its IC50 in LoVo cells and HT-29 cell lines. SN-38 induces a concentration-dependent formation of cleavable complexes, which is not significantly different in LoVo cells and HT-29 cell lines. Cell accumulation of Irinotecan is markedly different, reaching consistently higher levels in HT-29 cells than in LoVo cells. The lactone E-ring of Irinotecan and SN-38 hydrolyses reversibly in aqueous solutions, and the interconversion between the lactone and carboxylate forms is dependent on pH and temperature. Liver is primarily responsible for the activation of Irinotecan to SN-38. At equal concentrations of Irinotecan and SN-38 glucuronide, the rate of beta-glucuronidase-mediated SN-38 production is higher than that formed from Irinotecan in both tumour and normal tissue. Irinotecan is also converted to SN-38 in intestines, plasma and tumor tissues. Irinotecan is significantly more active in SCLC than in NSCLC cell lines, whereas no significant difference between histological types is observed with SN-38.
|
| In vivo |
In COLO 320 xenografts, Irinotecan induces a maximum growth inhibition of 92%. A single dose of Irinotecan significantly increases amounts of topoisomerase I covalently bound to DNA in stomach, duodenum, colon and liver. Concomitantly, the Irinotecan-treated group shows significantly higher amounts of DNA strand breaks in colon mucosa cells compared to the control group.
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | AMPK / p-AMPK / mTOR / p-mTOR / p70S6K / p-p70S6K NFκB p65 / phospho-NFκB p65 / NFκB p50 / IκBα / p27Kip1 TopI / pAKT / pMEK / pERK / p-p38 MAPK / pJNK2 |
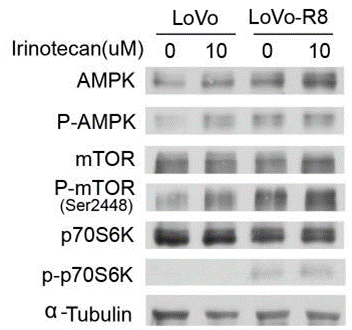
|
25973791 |
| Immunofluorescence | NFκB |
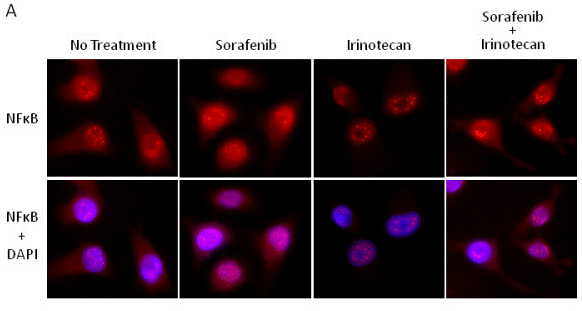
|
22206574 |
| Growth inhibition assay | Cell viability |
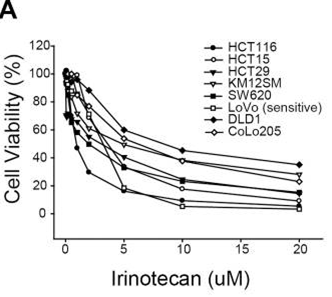
|
25973791 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05854498 | Recruiting | Metastatic Colorectal Cancer |
University of Wisconsin Madison|Ipsen |
October 13 2023 | Phase 2 |
| NCT05732129 | Not yet recruiting | Homologous Recombination Deficiency Alterations Metastatic Colorectal Cancer |
Fudan University |
March 1 2023 | Phase 2 |
| NCT05731518 | Recruiting | Small Cell Lung Cancer |
Biocity Biopharmaceutics Co. Ltd. |
February 23 2023 | Phase 1|Phase 2 |
| NCT06003998 | Recruiting | Colorectal Cancer|Peritoneal Metastases |
Catharina Ziekenhuis Eindhoven |
December 27 2022 | Phase 2 |
| NCT05277766 | Recruiting | Peritoneal Carcinomatosis|Peritoneal Metastases|Colorectal Cancer|Small Bowel Cancer|Appendix Cancer|Gastric Cancer|Pancreatic Cancer|Bile Duct Cancer |
University Hospital Ghent|Kom Op Tegen Kanker|University Ghent |
November 21 2022 | Phase 1 |
| NCT05379790 | Recruiting | Gastric Cancer|Peritoneal Metastases |
Erasmus Medical Center |
May 25 2022 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































