research use only
KD025 (Belumosudil) ROCK2 inhibitor
Cat.No.S7936
-chemical-structure-s7936.gif)
Chemical Structure
Molecular Weight: 452.51
Quality Control
Solubility
|
In vitro |
DMSO
: 90 mg/mL
(198.89 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 452.51 | Formula | C26H24N6O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 911417-87-3 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | SLx-2119 | Smiles | CC(C)NC(=O)COC1=CC=CC(=C1)C2=NC3=CC=CC=C3C(=N2)NC4=CC5=C(C=C4)NN=C5 | ||
Mechanism of Action
| Targets/IC50/Ki |
ROCK2
(Cell-free assay) 41 nM(Ki)
ROCK2
(Cell-free assay) 60 nM
|
|---|---|
| In vitro |
Belumosudil (KD025) down-regulates the IL-17 and IL-21 secretion in human PBMCs, and leads to down-regulation of STAT3 phosphorylation, IRF4, and RORγt expression in CD4+ T cells. It also inhibits the secretion of IL-21, IL-17, and IFNγ along with decreasing phosphorylated STAT3 and reduced protein expression of IRF4 and BCL6 in human peripheral blood mononuclear cells. |
| Kinase Assay |
Recombinant ROCK1 and ROCK2 assays
|
|
Belumosudil (KD025) compound dilutions and reactions are performed in 96-well polystyrene low-binding plates. Filtration is done in 96-well filter plates containing hydrophilic phospho-cellulose cation exchanger membranes. Enzymatic activity of the recombinant ROCK1 and ROCK2 is measured radiometrically in 50 μL of reaction mixture containing assay buffer (50 mmol/L Tris, pH 7.5, 0.1 mmol/L ethyleneglycoltetraacetic acid, 10 mmol/L magnesium acetate and 1 mmol/L dithiothreitol).. The reaction is incubated for 45 min at room temperature and stopped with 25 μL of 3% phosphoric acid. Phosphorylated long S6 peptide is separated from unreacted [[gamma]-33P]ATP by filtration of the quenched reaction contents through a P30 phosphocellulose filter plate. Each filter is washed three times with 75 μL of 75 mmol/L phosphoric acid and one time with 30 μL of 100% methanol. Filter plates are allowed to dry and 30 μL of OptiPhase ‘SuperMix’ scintillation fluid is added to each well. 33Phosphorous is quantified in an I450 MicroBeta scintillation counter and corrected by subtracting the radioactivity associated with the background samples. Data are analyzed and expressed as percent inhibition using the formula ((U − B)/(C − B)) × 100 where U is the unknown value, B is the average of staurosporine background wells, and C is the average of control wells. Curve fitting is performed by GraphPad Prism software using sigmoidal dose-response (variable slope) equation type analysis to generate IC50 values. Ki values are calculated from an equation of Ki = IC50/(1 + [S]/Km)), where [S] and Km are the concentration of ATP and the Km value of ATP, respectively.
|
|
| In vivo |
Belumosudil (KD025) (200 mg/kg, p.o.) inhibits ROCK activity in brain and heart, as measured by the degree of MYPT1 phosphorylation. It significantly reduces the area of perfusion defect and tissue loss in the ipsilateral hemisphere. In a collagen-induced arthritis (CIA) mouse model, this compound (200 mg/kg, i.p.) downregulates the progression of collagen-induced arthritis via targeting of the Th17-mediated pathway. It (150 mg/kg, i.p. or p.o.) effectively ameliorates cGVHD in a full MHC-mismatch model of multi-organ system cGVHD with bronchiolitis obliterans syndrome and a minor MHC-mismatch model of sclerodermatous GVHD. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | pSTAT3 / Bcl6 / pSTAT5 / Blimp1 |
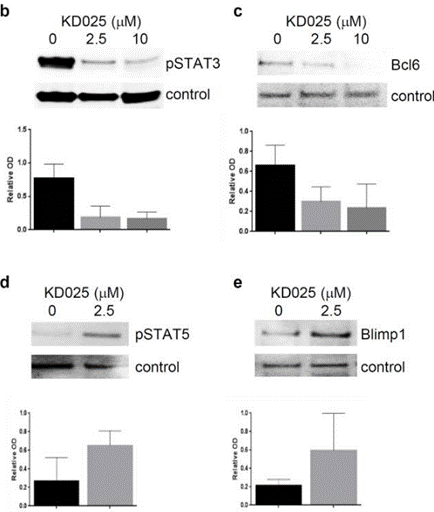
|
27436361 |
| ELISA | IL-21 / IL-17 / IFN-γ |
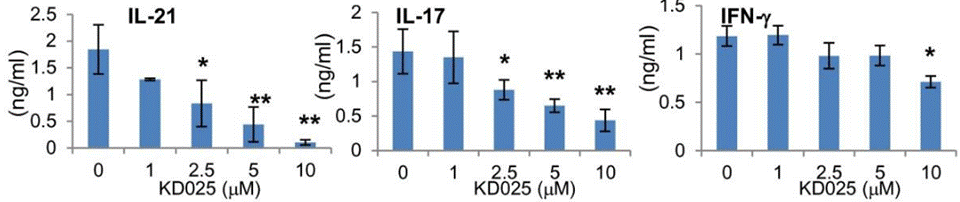
|
25385601 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03919799 | Terminated | System; Sclerosis|Diffuse Cutaneous Systemic Sclerosis |
Kadmon a Sanofi Company|Sanofi |
June 26 2019 | Phase 2 |
| NCT02841995 | Completed | Graft vs Host Disease |
Kadmon a Sanofi Company|Sanofi |
September 15 2016 | Phase 2 |
| NCT02557139 | Completed | Bioavailability |
Kadmon Corporation LLC|Quotient Clinical |
September 2015 | Phase 1 |
| NCT02317627 | Completed | Psoriasis Vulgaris |
Kadmon Corporation LLC |
December 2014 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































