research use only
Rigosertib (ON-01910) PLK inhibitor
Cat.No.S1362
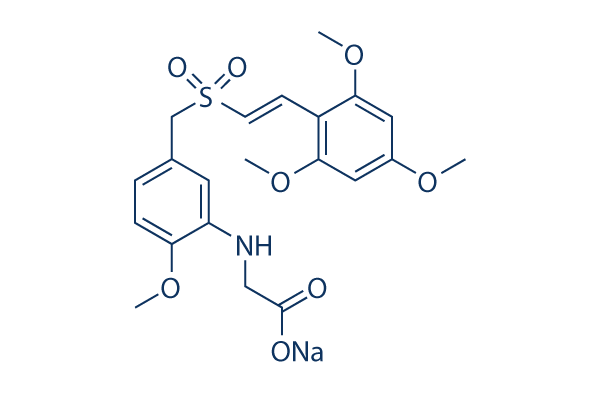
Chemical Structure
Molecular Weight: 473.47
Quality Control
| Related Targets | CDK HSP PD-1/PD-L1 ROCK Wee1 DNA/RNA Synthesis Microtubule Associated Ras KRas Aurora Kinase |
|---|---|
| Other PLK Inhibitors | Volasertib (BI6727) BI 2536 GSK461364 Onvansertib (NMS-1286937, NMS-P937) CFI-400945 Ro3280 HMN-214 SBE 13 HCl Centrinone (LCR-263) MLN0905 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| T47D | Cytotoxicity assay | 72 hrs | Cytotoxicity against human T47D cells after 72 hrs by MTT assay, GI50 = 0.01 μM. | 21463944 | ||
| MDA468 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MDA468 cells after 72 hrs by MTT assay, GI50 = 0.02 μM. | 21463944 | ||
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MCF7 cells after 72 hrs by MTT assay, GI50 = 0.05 μM. | 21463944 | ||
| HCT116 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HCT116 cells after 72 hrs by MTT assay, GI50 = 0.05 μM. | 21463944 | ||
| MDA468 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MDA468 cells after 48 hrs by MTT assay, GI50 = 0.302 μM. | 21463944 | ||
| MDA468 | Cytotoxicity assay | 24 hrs | Cytotoxicity against human MDA468 cells after 24 hrs by MTT assay, GI50 = 0.601 μM. | 21463944 | ||
| MRC5 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human MRC5 cells after 72 hrs by MTT assay, GI50 = 0.71 μM. | 21463944 | ||
| MCF7 | Function assay | 1 uM | Metabolic stability of the compound in human MCF7 cells at 1 uM | 21463944 | ||
| MRC5 | Function assay | 1 uM | Metabolic stability of the compound in human MRC5 cells at 1 uM | 21463944 | ||
| K562 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human K562 cells after 96 hrs by trypan blue exclusion assay, IC50 = 0.0075 μM. | 21812421 | ||
| DU145 | Cytotoxicity assay | 96 hrs | Cytotoxicity against human DU145 cells after 96 hrs by trypan blue exclusion assay, IC50 = 0.075 μM. | 21812421 | ||
| HeLa | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HeLa cells assessed as cell growth inhibition after 72 hrs by MTT assay, GI50 = 0.012 μM. | 24471873 | ||
| LNCAP | Antiproliferative assay | 72 hrs | Antiproliferative activity against AR positive human LNCAP cells assessed as cell growth inhibition after 72 hrs by MTT assay, GI50 = 0.025 μM. | 24471873 | ||
| PANC1 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human PANC1 cells assessed as cell growth inhibition after 72 hrs by MTT assay, GI50 = 0.039 μM. | 24471873 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against ER positive human MCF7 cells assessed as cell growth inhibition after 72 hrs by MTT assay, GI50 = 0.05 μM. | 24471873 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells assessed as cell growth inhibition after 72 hrs by MTT assay, GI50 = 0.05 μM. | 24471873 | ||
| MDA-MB-231 | Antiproliferative assay | 72 hrs | Antiproliferative activity against ER negative human MDA-MB-231 cells assessed as cell growth inhibition after 72 hrs by MTT assay, GI50 = 0.057 μM. | 24471873 | ||
| A2780 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human A2780 cells assessed as cell growth inhibition after 72 hrs by MTT assay, GI50 = 0.062 μM. | 24471873 | ||
| HCT116 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT116 cells assessed as cell growth inhibition after 72 hrs by MTT assay, GI50 = 0.07 μM. | 24471873 | ||
| DU145 | Antiproliferative assay | 72 hrs | Antiproliferative activity against AR negative human DU145 cells assessed as cell growth inhibition after 72 hrs by MTT assay, GI50 = 0.075 μM. | 24471873 | ||
| A2780 | Function assay | 0.25 uM | 24 hrs | Reduction in CDC25C level in human A2780 cells at 0.25 uM after 24 hrs by Western blot analysis | 24471873 | |
| A2780 | Apoptosis assay | 0.25 uM | 24 hrs | Induction of apoptosis in human A2780 cells assessed as caspase-3/7 activation at 0.25 uM after 24 hrs by using Apo-ONE homogeneous caspase-3/7 kit | 24471873 | |
| A2780 | Function assay | 0.25 uM | 24 hrs | Reduction in Mcl1 level in human A2780 cells at 0.25 uM after 24 hrs by Western blot analysis | 24471873 | |
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 94 mg/mL
(198.53 mM)
Water : 94 mg/mL Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 473.47 | Formula | C21H24NNaO8S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1225497-78-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | COC1=C(C=C(C=C1)CS(=O)(=O)C=CC2=C(C=C(C=C2OC)OC)OC)NCC(=O)[O-].[Na+] | ||
Mechanism of Action
| Targets/IC50/Ki |
PLK1
(Cell-free assay) 9 nM
PDGFR
(Cell-free assay) 18 nM
Bcr-Abl
(Cell-free assay) 32 nM
Flt1
(Cell-free assay) 42 nM
Src
(Cell-free assay) 155 nM
Fyn
(Cell-fre assay) 182 nM
CDK1
(Cell-free assay) 260 nM
PLK2
(Cell-free assay) 260 nM
|
|---|---|
| In vitro |
Rigosertib (ON-01910) is a non-ATP-competitive inhibitor to PLK1 with IC50 of 9 nM. It also exhibits inhibition against PLK2, PDGFR, Flt1, BCR-ABL, Fyn, Src, and CDK1, with IC50 of 18-260 nM. This compound shows cell killing activity against 94 different tumor cell lines with IC50 of 50-250 nM, including BT27, MCF-7, DU145, PC3, U87, A549, H187, RF1, HCT15, SW480, and KB cells. While in normal cells, such as HFL, PrEC, HMEC, and HUVEC, it has little or no effect unless its concentration is greater than 5-10 μM. In HeLa cells, Rigosertib (100-250 nM) induces spindle abnormalities and apoptosis. It also inhibits several multidrug resistant tumor cell lines, including MES-SA, MES-SA/DX5a, CEM, and CEM/C2a, with IC50 of 50-100 nM. In DU145 cells, this compound (0.25-5 μM) blocks cell cycle progression in G2/M phase, results in an accumulation of cells containing subG1 content of DNA, and activates apoptotic pathways. In A549 cells, it (50 nM-0.5 μM) induces loss of viability and caspase 3/7 activation. In a recent study, Rigosertib induces apoptosis in chronic lymphocytic leukemia (CLL) cells without toxicity against T-cells or normal B-cells. It also abrogates the pro-survival effect of follicular dendritic cells on CLL cells and reduces SDF-1-induced migration of leukemic cells.
|
| Kinase Assay |
In vitro enzyme assays for PLK1
|
|
Recombinant PLK1 (10 ng) is incubated with different concentrations of Rigosertib (ON-01910) in a 15 µL reaction mixture (50 mM HEPES, 10 mM MgCl2, 1 mM EDTA, 2 mM Dithiothreitol, 0.01% NP-40 [pH 7.5]) for 30 min at room temperature. Kinase reactions are performed for 20 min at 30 °C in a volume of 20 µL (15 µL enzyme + this compound, 2 µL 1 mM ATP), 2 µL of γ32P-ATP (40 μCi), and 1 µL of recombinant Cdc25C (100 ng) or casein (1 μg) substrates. Reactions are terminated by boiling for 2 min in 20 µL of 2× Laemmli buffer. Phosphorylated substrates are separated by 18% SDS-PAGE. The gels are dried and exposed to X-ray film for 3-10 min.
|
|
| In vivo |
In mouse xenograft models of Bel-7402, MCF-7, and MIA-PaCa cells, Rigosertib (ON-01910) (250 mg/kg) markedly inhibits tumor growth. This compound (200 mg/kg) also shows inhibition on tumor growth in a mouse xenograft model of BT20 cells.
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
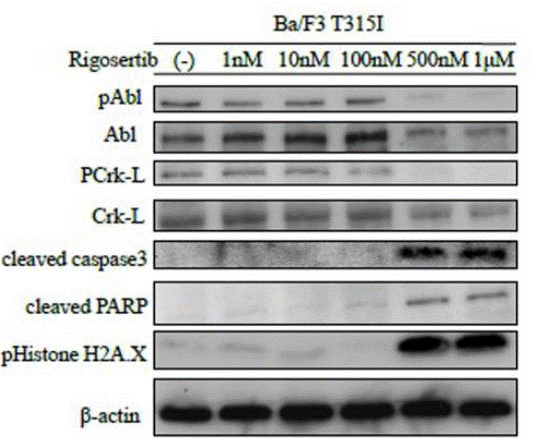
| Western blot | pAbl / Abl / PCrk-L / Crk-L / Cleaved caspase3 / Cleaved PARP / pHistone H2A.X |
|
26008977 |
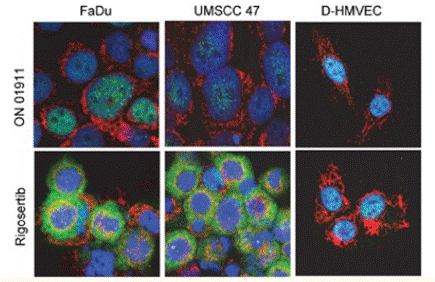
| Immunofluorescence | p-ATF / COX IV |
|
27764820 |
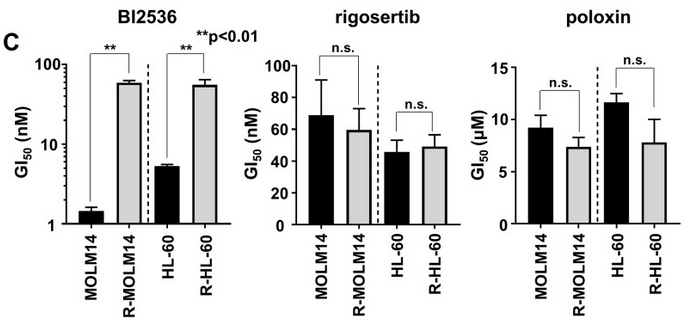
| Growth inhibition assay | GI50 Cell viability |
|
29108241 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04177498 | Recruiting | Recessive Dystrophic Epidermolysis Bullosa |
Thomas Jefferson University|Traws Pharma Inc. |
August 24 2021 | Early Phase 1 |
| NCT02075034 | Withdrawn | Myelodysplastic Syndrome |
Traws Pharma Inc. |
May 2014 | Phase 1 |
| NCT02030639 | Completed | Healthy |
Traws Pharma Inc. |
January 2014 | Phase 1 |
| NCT01928537 | Completed | Myelodysplastic Syndromes|Refractory Anemia With Excess Blasts|Chronic Myelomonocytic Leukemia|Cytopenia |
Traws Pharma Inc. |
August 2013 | Phase 3 |
| NCT01807546 | Completed | Head and Neck Squamous Cell Carcinoma|Anal Squamous Cell Carcinoma|Lung Squamous Cell Carcinoma|Cervical Squamous Cell Carcinoma|Esophageal Squamous Cell Carcinoma|Skin Squamous Cell Carcinoma|Penile Squamous Cell Carcinoma |
Traws Pharma Inc. |
March 2013 | Phase 2 |
| NCT01168011 | Completed | Solid Tumor |
Traws Pharma Inc. |
July 2010 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































