research use only
Raloxifene HCl Estrogen/progestogen Receptor modulator
Cat.No.S1227
Chemical Structure
Molecular Weight: 510.04
Quality Control
| Related Targets | Adrenergic Receptor GPR Androgen Receptor Glucocorticoid Receptor ACE RAAS Progesterone Receptor Opioid Receptor PGES THR |
|---|---|
| Other Estrogen/progestogen Receptor Inhibitors | Elacestrant (RAD1901) Dihydrochloride Vepdegestrant (ARV-471) MPP dihydrochloride Kaempferol Cholesterol G15 Endoxifen HCl Chrysin Licochalcone A AZD9496 |
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(196.06 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 510.04 | Formula | C28H27NO4S.HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 82640-04-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | LY156758 (Keoxifene) HCl | Smiles | C1CCN(CC1)CCOC2=CC=C(C=C2)C(=O)C3=C(SC4=C3C=CC(=C4)O)C5=CC=C(C=C5)O.Cl | ||
Mechanism of Action
| Features |
Raloxifene is as effective as tamoxifen in reducing the incidence of breast cancer in postmenopausal women at increased risk.
|
|---|---|
| Targets/IC50/Ki |
ER
(Cell-free assay) 5.7 nM
|
| In vitro |
Raloxifene, has been demonstrated as a potent uncompetitive inhibitor of human liver aldehyde oxidase-catalyzed oxidation of phthalazine, vanillin, and nicotine-Delta1'(5')-iminium ion, with Ki values of 0.87 nM, 1.2 nM and 1.4 nM. Raloxifene has also been shown to be a noncompetitive inhibitor of an aldehyde oxidase-catalyzed reduction reaction of a hydroxamic acid-containing compound, with a Ki of 51 nM. Raloxifene activates TGF beta 3 promoter as a full agonist at nanomolar concentrations, and raloxifene inhibits the estrogen response element-containing vitellogenin promoter expression as a pure estrogen antagonist in transient transfection assays. |
| In vivo |
Raloxifene restores both bone mineral density and TGF beta 3 messenger RNA expression in the femur to levels measured in intact rats. Raloxifene (0.1 mg/kg-10 mg/kg, orally for 5 weeks) increases bone mineral density in the distal femur and proximal tibia in ovariectomized (OVX) rat. Raloxifene reduces serum cholesteroloral with ED50 of 0.2 mg/kg in ovariectomized (OVX) rat. Raloxifene diverges dramatically from estrogen in its lack of significant estrogenic effects on uterine tissue. Raloxifene prevents cancellous osteopenia as well as the changes in radial bone growth, bone resorption, and blood cholesterol, but is less effective in reducing cancellous bone formation and does not prevent uterine atrophy in ovariectomized (OVX) rats. Raloxifene (3 mg/kg/day) has potent estrogenic activity on bone resorption and serum cholesterol, a lesser effect on bone formation, and minimal activity on uterine wet weight in ovariectomized (OVX) rats. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | p-STAT3 / STAT3 / Survivin / Bcl-2 / Bcl-xl RhoA / Rock I / Rock II / p-MLC / MLC |
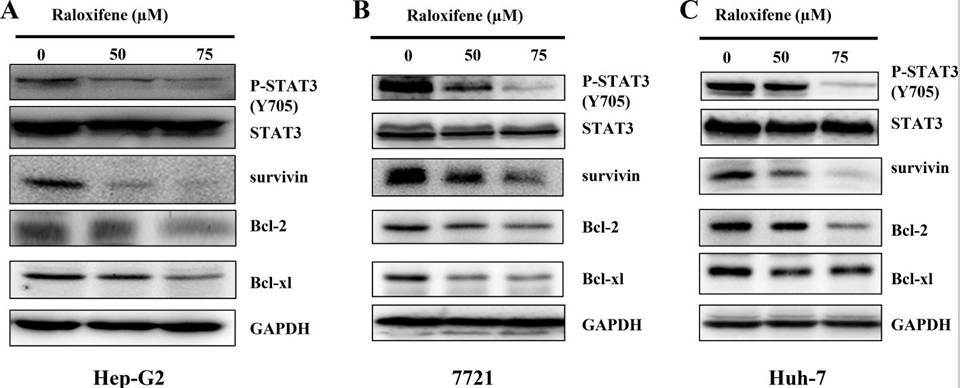
|
28430601 |
| Growth inhibition assay | Cell viability |
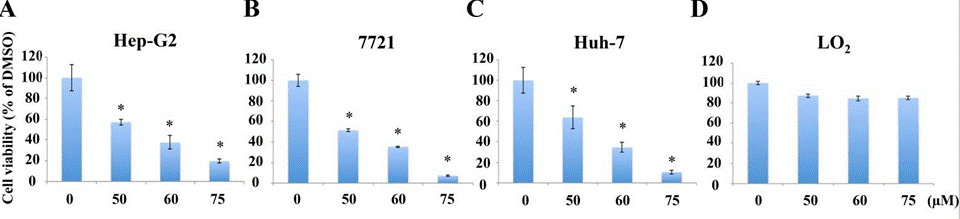
|
28430601 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT01973738 | Unknown status | Selective Estrogen Receptor Modulator |
Toshihiko Kono|Tomidahama Hospital |
January 2012 | -- |
| NCT02977949 | Unknown status | Selective Estrogen Receptor Modulator (SERM) |
Toshihiko Kono|Tomidahama Hospital |
January 2012 | -- |
| NCT01573637 | Completed | Schizophrenia in Post Menopausal Women |
Fundació Sant Joan de Déu|Hospital Sant Joan de Deu|Parc Sanitari Sant Joan de Déu|Stanley Medical Research Institute |
July 2011 | Phase 3 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































