research use only
PMSF Serine Protease inhibitor
Cat.No.S3025
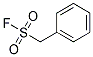
Chemical Structure
Molecular Weight: 174.19
Quality Control
| Related Targets | HDAC Caspase Proteasome Secretase MMP HCV Protease Cysteine Protease DPP Tyrosinase HIV Protease |
|---|---|
| Other Serine Protease Inhibitors | Leupeptin Hemisulfate Nafamostat mesilate (FUT-175) Sivelestat AEBSF HCl Gabexate Mesylate Alvelestat (AZD9668) Sivelestat sodium tetrahydrate UK-371804 HCl Ulinastatin Fulacimstat |
Solubility
|
In vitro |
DMSO
: 35 mg/mL
(200.93 mM)
Ethanol : 35 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 174.19 | Formula | C7H7FO2S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 329-98-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Phenylmethylsulfonyl Fluoride, Benzylsulfonyl fluoride | Smiles | C1=CC=C(C=C1)CS(=O)(=O)F | ||
Mechanism of Action
| Targets/IC50/Ki |
cysteine protease
chymotrypsin
|
|---|---|
| In vitro |
Although human trypsin is less susceptible to inhibition by PMSF, this compound rapidly inactivates purified chymotrypsin from human pancreas. It also rapidly inhibits acetylcholinesterase from human red cells. As an inhibitor of phosphatidylinositol-specific phospholipase C, treatment with this chemical at 2 mM almost completely inhibits carbachol-stimulated inositol incorporation into phosphatidylinositol (PI) of longitudinal smooth muscle of guinea pig ileum, while it has no effect on potassium-stimulated inositol incorporation. In contrast to its specific inhibition of carbachol-stimulated phosphoinositide turnover, this agent produces a transient inhibition of contraction by both carbachol and potassium. It has been shown to inhibit the addition of ethanolamine phosphate to glycosylphosphatidylinositol (GPI) intermediates in Trypanosoma brucei. This inhibitor also inhibits the acylation of the inositol residue of GPI intermediates in bloodstream form T. brucei. It inhibits ethanolamine phosphate addition and inositol acylation for procyclic forms of T. brucei but not for mammalian HeLa cells. This compound is the more reactive inactivator of mouse acetylcholinesterase (AChE), as the 8-fold higher BSF concentration is necessary to achieve even a 6-fold slower inactivation than that using PMSF.
|
| In vivo |
Intraperitoneal injection of PMSF produces dose-dependent analgesia in Sprague-Dawley rats. This compound significantly enhances the analgesic effect of beta-endorphin (END) in rats. Mice receiving i.p. injections of this chemical exhibit cannabinoid effects that include antinociception, hypothermia and immobility with ED50 of 86 mg/kg, 224 mg/kg and 206 mg/kg, respectively. Pretreatment with an inactive dose of this compound (30 mg/kg) enhances the effects of anandamide on tail-flick response (antinociception), spontaneous activity and mobility by 5-, 10- and 8-fold, respectively. Administration of this chemical 12 hours prior to PSP causes complete protection in organophosphorus ester-induced delayed neuropathy (OPIDN) in hens, but it administered 4 hours after PSP potentiates its neurotoxic effects. Pretreatment with this compound (30 mg/kg, i.p.) prior to an injection of 1 or 10 mg/kg 3H-anandamide results 5 minutes later in enhanced brain levels of anandamide compared to those obtained with 3H-anandamide plus vehicle injection. Pretreatment with it inhibits tri-ortho-cresyl phosphate (TOCP)-induced neurofilament (NF) degradation, and protects hens against the development of organophosphate-induced delayed neuropathy (OPIDN). Administration of this chemical enhances the characteristic cannabimimetic effects of Δ(9)-tetrahydrocannabinol (THC) or anandamide (AEA) in ICR mice, by inhibiting the enzyme fatty acid amide hydrolase.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04062786 | Unknown status | Scheduled Heart Surgery|Valve Replacement|Coronary Artery Bypass |
University Hospital Strasbourg France |
February 21 2019 | -- |
| NCT03300323 | Unknown status | Peri-operative Fluid Management |
St George''s Healthcare NHS Trust |
October 2017 | Not Applicable |
| NCT02569008 | Completed | Post Cardiac Surgery |
St George''s University of London |
January 2014 | Not Applicable |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































