- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Savolitinib (AZD6094) c-Met inhibitor
Cat.No.S7674
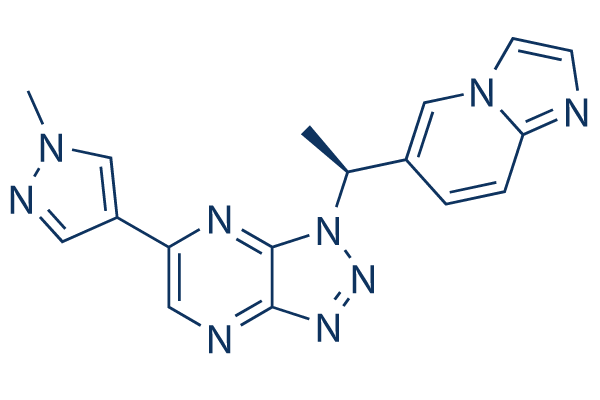
Chemical Structure
Molecular Weight: 345.36
Jump to
Quality Control
Batch:
Purity:
99.95%
99.95
| Related Targets | EGFR VEGFR PDGFR FGFR Src MEK CSF-1R FLT3 HER2 c-Kit |
|---|---|
| Other c-Met Products | Tepotinib Dihexa SGX-523 Foretinib PHA-665752 SU11274 BMS-777607 JNJ-38877605 Tivantinib PF-04217903 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| NCI-H441 | Function assay | 1 h | Inhibition of c-Met autophosphorylation in human NCI-H441 cells for 1 hr by ELISA, IC50=0.003 μM | 25148209 | ||
| NCI-H441 | Proliferation assay | 72 h | Antiproliferative activity against human NCI-H441 cells assessed as HGF-induced proliferation after 72 hrs by MTT assay, IC50=0.006 μM | 25148209 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 69 mg/mL
(199.79 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 345.36 | Formula | C17H15N9 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1313725-88-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | HMPL-504, Volitinib | Smiles | CC(C1=CN2C=CN=C2C=C1)N3C4=NC(=CN=C4N=N3)C5=CN(N=C5)C | ||
Mechanism of Action
| Targets/IC50/Ki |
p-Met
(Cell-free assay) 3 nM
c-Met
(Cell-free assay) 5 nM
|
|---|---|
| In vitro |
Volitinib has exquisite kinase selectivity and excellent potency. Volitinib displays a highly selective profile across a gastric cell line panel, potently inhibiting cell growth only in those lines with dysregulated cMET (EC50 values 0.6 nM/L-12.5 nM/L). Volitinib has high membrane permeability without efflux transport across Caco-2 cell monolayer and exhibits negligible P-gp inhibition (IC50 > 17 μM). Volitinib shows no significant reversible or mechanism-based CYP inhibition in human liver microsomes, and no induction of CYP1A2 and CYP3A4 in human hepatocytes. |
| In vivo |
In a mouse pharmacokinetic study (male ICR mice), the clearance of the compound is 4.28 L/(h·kg) and the half-time is 1.7 h. Despite its moderate oral bioavailability (F = 27.2%), the overall plasma exposure is much higher. Volitinib demonstrates dose-dependent tumor growth inhibition in a U87MG subcutaneous xenograft model. Its treatment leads to pharmacodynamic modulation of c-MET signaling and potent tumor stasis in 3/3 cMET-dysregulated gastric cancer patient-derived tumor xenograft models, but has negligible activity in a gastric cancer control model. Volitinib has moderate plasma protein binding rate (60%∼70% in rat, dog, and human; 40% in mouse; 80% in monkey) and exhibits wide distribution to different organs in rat, with high exposures in liver and kidney, very low in brain, spinal cord and testis compared to the plasma level. In PK studies in mouse, rat and dog, Volitinib shows the rapid oral absorption (Tmax<2.5 h) with high exposures and the acceptable bioavailability at 27.2%, 42.6% and 86.3%, respectively. The in vivo clearance (CL) is 11.0, 11.8 and 3.5 mL/min/kg in mouse, rat and dog, respectively, revealing a low extraction ratio. The volume of distribution in steady state (Vss) is 0.4, 1.4 and 1.4 L/kg in those species, respectively, indicating a moderate to low distribution pattern. Volitinib also displays linear pharmacokinetics (PK) in the dose ranges of 1 to 25 mg/kg in rat and 2 to 10 mg/kg in dog. Food hardly affects its PK profile in dog. In contrast, volitinib in monkey shows a notably high extraction ratio (CL=17.2 mL/min/kg) consistent with the in vitro metabolism result. Considering the rapid absorption of volitinib (Tmax=1.9 h) and moderately low distribution (Vss=0.7 L/kg), the poor oral bioavailability (1.9%) of volitinib in monkey is considered to be the result of excessive first-pass extraction. Overall, volitinib exhibits favorable preclinical PK/ADME properties. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | pMET / MET / pAKT / AKT / pERK / ERK |
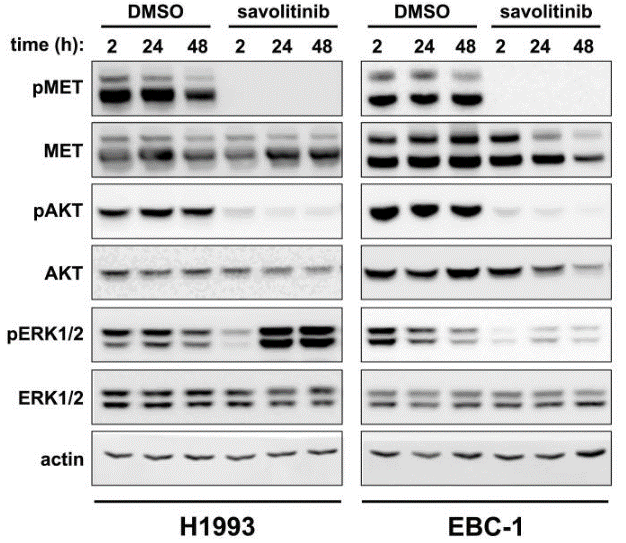
|
27472392 |
| Growth inhibition assay | Cell viability |
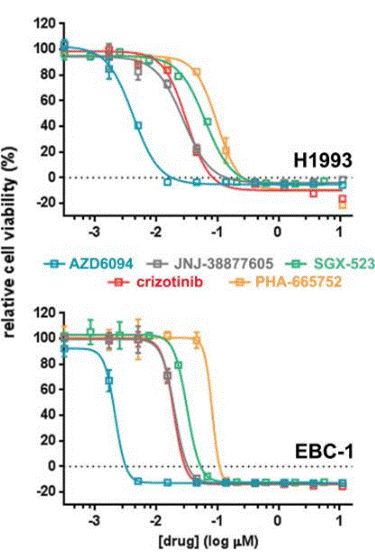
|
27472392 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05888207 | Completed | Healthy Male Subjects |
AstraZeneca|Parexel |
June 2 2023 | Phase 1 |
| NCT05043090 | Recruiting | Papillary Renal Cell Carcinoma |
AstraZeneca |
October 28 2021 | Phase 3 |
| NCT04606771 | Active not recruiting | Non-Small Cell Lung Cancer |
AstraZeneca |
September 28 2020 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































