research use only
Guanabenz Acetate Adrenergic Receptor agonist
Cat.No.S4065
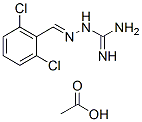
Chemical Structure
Molecular Weight: 291.13
Quality Control
| Related Targets | AChR 5-HT Receptor COX Calcium Channel Histamine Receptor Dopamine Receptor GABA Receptor TRP Channel Cholinesterase (ChE) GluR |
|---|---|
| Other Adrenergic Receptor Inhibitors | ICI 118551 Hydrochloride (Zenidolol) L755507 Yohimbine HCl Atipamezole Higenamine hydrochloride Detomidine HCl Naftopidil Demethyl-Coclaurine Buflomedil HCl Fenoterol hydrobromide |
Solubility
|
In vitro |
DMSO
: 15 mg/mL
(51.52 mM)
Ethanol : 15 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 291.13 | Formula | C8H8Cl2N4.C2H4O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 23256-50-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | WY-8678 Acetate | Smiles | CC(=O)O.C1=CC(=C(C(=C1)Cl)C=NN=C(N)N)Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
α2a-adrenergic receptor
8.25(pEC50)
α2b-adrenergic receptor
7.01(pEC50)
α2c-adrenergic receptor
<5(pEC50)
|
|---|---|
| In vitro |
Guanabenz at a concentration of 30 μM causes a time-dependent loss of nNOS-activity, which is present in cytosol prepared from rat nNOS transfected HEK 293 cells, with a Ki of 1 μM. Guanabenz can induce a concentration-dependent loss of nNOS activity. Within the first 3 h of treatment, 50 μM Guanabenz decreases the accumulation of nitrite and nitrate by approximately 75% in cells. Treatment of HEK 293 cells for 24 h with Guanabenz (100 μM) causes an approximately 35% decrease in the amount of immunodetectable nNOS protein. Guanabenz enhances proteolysis of the protein with the half-life decreasing by one-half from 20 to 10 h.
Guanabenz is active against [ PSI+] prion in the yeast-based assay independent of its agonist activity on α2-adrenergic receptors. Guanabenz (10 μM) promote complete PrPSc clearance in mammalian MovS6 cell-based assay.
|
| In vivo |
Guanabenz injected i.v. in doses of 0.1 mg/kg causes an initial increase in blood pressure followed by a prolonged fall and a decrease in cardiac output, contractile force and heart rate in anesthetized dogs, which requires the presence of sympathetic tone. Guanabenz inhibits pressor responses to various procedures which initiate general sympathetic discharge. Guanabenz also antagonizes responses to sympathetic nerve stimulation. Guanabenz injected at doses of 0.5 mg/kg and above lowers blood pressure and heart rate in unanesthetized hypertensive rats and dogs.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02423083 | Terminated | Multiple Sclerosis Relapsing-Remitting|Multiple Sclerosis |
National Institute of Neurological Disorders and Stroke (NINDS)|National Institutes of Health Clinical Center (CC) |
April 21 2015 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































