research use only
Naftopidil Adrenergic Receptor antagonist
Cat.No.S2126
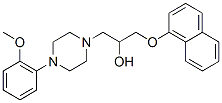
Chemical Structure
Molecular Weight: 392.49
Quality Control
Solubility
|
In vitro |
DMSO
: 17 mg/mL
(43.31 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 392.49 | Formula | C24H28N2O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 57149-07-2 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | KT-611 | Smiles | COC1=CC=CC=C1N2CCN(CC2)CC(COC3=CC=CC4=CC=CC=C43)O | ||
Mechanism of Action
| Targets/IC50/Ki |
α1D-adrenergic receptor
1.2 nM(Ki)
α1A-adrenergic receptor
3.7 nM(Ki)
α1B-adrenergic receptor
20 nM(Ki)
|
|---|---|
| In vitro |
Naftopidil is selective for the alpha1d-adrenoceptor with approximately 3- and 17-fold higher affinity than for the alpha1a- and alpha1b-adrenoceptor subtypes, respectively. This compound has growth inhibitory effect in androgen-sensitive and -insensitive human prostate cancer cell lines. It induces p21(cip1) but not p27(kip1) in PC-3 cells. This chemical induces apoptosis in all the investigated malignant mesothelioma cells, and a similar effect is obtained with prazosin, another α1-adrenoceptor blocker. Its-induced reduction in cell viability is inhibited by GF109203X, while prazosin-induced in cell viability is less affected. This compound, an alpha 1-adrenoreceptor antagonist produces a concentration-dependent inhibition ofcollagen-induced Ca2+ mobilization, maximum inhibition (22.9%) occurring with 40 mM of it. It also inhibits the adrenaline-induced rise in [Ca2+]i in a concentration-dependent manner (30 mM doxazosin), significant inhibitions of platelet aggregation also being produced. This chemical (0.3, 1, and 3 μM) inhibits 5-HT-induced bladder contraction in a concentration-dependent manner. It inhibits both the 5-HT(2A) and 5-HT(2) receptor agonists-induced bladder contractions. This compound binds to the human 5-HT(2A) and 5-HT(2B) receptors with pKi values of 6.55 and 7.82, respectively.
|
| In vivo |
Naftopidil selectively inhibits the phenylephrine-induced increase in prostatic pressure compared with mean blood pressure in the anesthetized dog model. This compound inhibits 5-HT-induced bladder contraction via blockade of the 5-HT(2A) and 5-HT(2B) receptors in rats.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT00967772 | Completed | Healthy |
Dong-A ST Co. Ltd. |
September 2009 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































