research use only
Droxidopa Adrenergic Receptor agonist
Cat.No.S3041
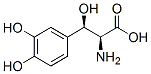
Chemical Structure
Molecular Weight: 213.19
Quality Control
| Related Targets | CXCR Hedgehog/Smoothened PKA AChR 5-HT Receptor Histamine Receptor Dopamine Receptor Ras KRas GPR |
|---|---|
| Other Adrenergic Receptor Inhibitors | Zenidolol (ICI-118551) Hydrochloride Yohimbine HCl L755507 Atipamezole Higenamine hydrochloride Fenoterol hydrobromide Medetomidine HCl Rauwolscine hydrochloride Deoxycorticosterone acetate Detomidine HCl |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| HepG2 cells | Function assay | 24 h | Activation of human PXR expressed in human HepG2 (DPX-2) cells assessed as induction of CYP3A4 after 24 hrs by luminescent analysis, EC50=0.004 μM | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 2 mg/mL
(9.38 mM)
Water : 2 mg/mL Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 213.19 | Formula | C9H11NO5 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 23651-95-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | L-DOPS | Smiles | C1=CC(=C(C=C1C(C(C(=O)O)N)O)O)O | ||
Mechanism of Action
| Features |
An artificial amino acid.
|
|---|---|
| Targets/IC50/Ki |
Adrenergic Receptor
|
| In vitro |
Droxidopa is a pro-drug which has a structure similar to noradrenaline, but with a carboxyl group. It can be administered orally, unlike noradrenaline, and after absorption is converted by the enzyme dopa decarboxylase into noradrenaline thus increasing levels of the neurotransmitter which is identical to endogenous noradrenaline. This compound is well tolerated. It could exert its pressor effect in three different ways: a) as a central stimulator of sympathetic activity; b) as a peripheral sympathetic neurotransmitter; c) as a circulating hormone. This chemical taken alone increases standing blood pressure. It can also cross the blood–brain barrier (BBB) where it is converted to norepinephrine and epinephrine from within the brain.
|
| In vivo |
The acute administration of droxidopa in PVL and BDL rats caused a significant and maintained increase in arterial pressure and mesenteric arterial resistance, with a significant decrease of mesenteric arterial and portal blood flow, without changing portal pressure and renal blood flow. This compound-treated rats also showed a decreased ratio of p-eNOS/eNOS and p-AKT/AKT and increased activity of RhoK in SMA.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03446807 | Withdrawn | Parkinson Disease|Multiple System Atrophy|Progressive Supranuclear Palsy |
Loma Linda University|H. Lundbeck A/S |
December 2021 | Phase 2 |
| NCT04977388 | Recruiting | Menkes Disease|Occipital Horn Syndrome |
Stephen G. Kaler MD|Nationwide Children''s Hospital |
July 12 2021 | Phase 1|Phase 2 |
| NCT03173781 | Completed | Parkinson''s Disease |
Colorado Springs Neurological Associates|H. Lundbeck A/S |
April 2016 | Not Applicable |
| NCT01331122 | Withdrawn | Gait Disorders Neurologic |
Chelsea Therapeutics |
April 2012 | Phase 1|Phase 2 |
| NCT01354158 | Completed | Spinal Cord Injury|Hypotension |
Bronx VA Medical Center|Chelsea Therapeutics |
May 2011 | Phase 1 |
| NCT01327066 | Completed | QTc Interval |
Chelsea Therapeutics |
March 2011 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































