- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
research use only
Darifenacin HBr AChR antagonist
Darifenacin HBr (UK-88525) is a selective M3 muscarinic receptor antagonist with pKi of 8.9.
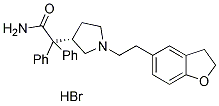
Chemical Structure
Molecular Weight: 507.46
Purity & Quality Control
Batch:
Purity:
99.99%
99.99
Related Products
| Related Targets | mAChR nAChR AChE | Click to Expand |
|---|---|---|
| Related Products | PNU-120596 Benzethonium Chloride Arecoline HBr Otilonium Bromide Lycorine Hyoscyamine (-)-Huperzine A (HupA) Nitenpyram Cytisine Chelidonine | Click to Expand |
| Related Compound Libraries | FDA-approved Drug Library Natural Product Library Neuronal Signaling Compound Library CNS-Penetrant Compound Library Anti-alzheimer Disease Compound Library | Click to Expand |
Signaling Pathway
Cell Culture and Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| HEK cells | Function assay | Agonist activity at human beta2-adrenoceptor expressed in HEK cells assessed as increase of cAMP level after 10 mins by radioimmunoassay, EC50=0.00082 μM | ||||
| H292 cells | Function assay | Agonist activity at human adrenergic beta2 receptor expressed in H292 cells assessed as stimulation of cAMP accumulation after 60 mins, EC50=0.01585 μM | ||||
| HEK293 cells | Function assay | Agonist activity at human beta2 adrenergic receptor expressed in HEK293 cells assessed as cAMP accumulation using [125I]cAMP by scintillation counting, EC50=0.0007943 μM | ||||
| CHO-K1 cells | Function assay | Inhibition of fast sodium current (INa) in Chinese Hamster Ovary (CHO) K1 cells transfected with human Nav1.5 measured using IonWorks Quattro automated patch clamp platform, IC50=1.58489 μM | ||||
| PC3 | Proliferation assay | 10 μM and 20 μM | blockade of CHRM3 by darifenacin could effectively reduce cell proliferation | |||
| 22Rv1 | Proliferation assay | 10 μM and 20 μM | blockade of CHRM3 by darifenacin could effectively reduce cell proliferation | |||
| H1299 | Proliferation assay | 0.3–100 µM | 72 h | significantly inhibited H1299 cell proliferation in a concentration-dependent manner | ||
| Click to View More Cell Line Experimental Data | ||||||
Mechanism of Action
| Targets |
|
|---|
In vitro |
||||
| In vitro | Darifenacin exerts non-parallel rightward displacement of the agonist curve and also significant depression of the maximum response (+)-cis-Dioxolane produced concentration-dependent contraction of the isolated bladder of rat. [1] Darifenacin produces a concentration dependent increase in R123 (P-gp probe) accumulation in MDCK cells. Darifenacin stimulates ATPase activity in P-gp membrane in a clear concentration dependent response manner with an estimated ED50 value of 1.6 µM. Darifenacin (100 nM) shows a significantly greater permeability for darifenacin in the basolateral to apical direction resulting in an efflux ratio in BBMEC monolayers of approximately 2.6. [2] | |||
|---|---|---|---|---|
In Vivo |
||
| In vivo | Darifenacin produces dose-dependent inhibition of amplitude of volume-induced bladder contractions(VIBCAMP), producing 35% inhibition at dose of 283.3 nmol/kg and maximal inhibition of approximately 50–55%. [1] Darifenacin (0.1 mg/kg i.v.) reduces bladder afferent activity in both Aδ and C fibers in female Sprague-Dawley rats, the decrease in afferent spikes in C fibers may be more pronounced than that in Aδ fibers. [3] Darifenacin (7.5 mg and 15 mg, daily) reduces the number of incontinence episodes per week from baseline by 67.7% and 72.8% respectively compared with 55.9% with placebo in patients with overactive bladder (OAB). Darifenacin (7.5 mg and 15 mg, daily) also shows significantly superior to placebo for improvements in micturition frequency, bladder capacity, frequency of urgency, severity of urgency and number of incontinence episodes leading to a change in clothing or pads in patients with overactive bladder (OAB). [4] | |
|---|---|---|
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT00703703 | Completed | Healthy |
Novartis|Procter and Gamble |
May 2008 | Phase 1 |
| NCT00921245 | Completed | Overactive Bladder |
Bayer |
June 2007 | -- |
| NCT00413790 | Completed | Healthy |
Novartis|Procter and Gamble |
November 2006 | Phase 4 |
| NCT00413426 | Completed | Healthy |
Novartis|Procter and Gamble |
June 2006 | Phase 1 |
| NCT00366002 | Completed | Overactive Bladder (OAB) |
Novartis|Procter and Gamble |
June 2006 | Phase 4 |
References |
|
Chemical Information
| Molecular Weight | 507.46 | Formula | C28H30N2O2.HBr |
| CAS No. | 133099-07-7 | SDF | Download SDF |
| Synonyms | UK-88525 | ||
| Smiles | C1CN(CC1C(C2=CC=CC=C2)(C3=CC=CC=C3)C(=O)N)CCC4=CC5=C(C=C4)OCC5.Br | ||
Storage and Stability
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 117 mg/mL ( (230.56 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Water : Insoluble Ethanol : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
|||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field