research use only
Acotiamide AChR inhibitor
Cat.No.S5075
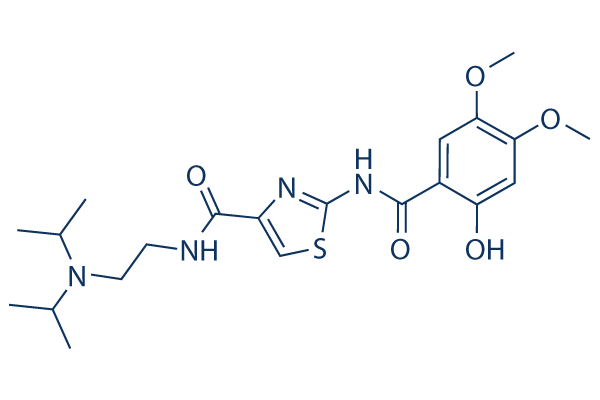
Chemical Structure
Molecular Weight: 450.55
Quality Control
Solubility
|
In vitro |
DMSO
: 90 mg/mL
(199.75 mM)
Ethanol : 38 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 450.55 | Formula | C21H30N4O5S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 185106-16-5 | -- | Storage of Stock Solutions |
|
|
| Synonyms | Acofide, Z388 | Smiles | CC(C)N(CCNC(=O)C1=CSC(=N1)NC(=O)C2=CC(=C(C=C2O)OC)OC)C(C)C | ||
Mechanism of Action
| Targets/IC50/Ki |
AChE
3 μM
|
|---|---|
| In vitro |
Acotiamide inhibited recombinant human and canine stomach-derived acetylcholinesterase (AChE) activity in vitro. The mode of the AChE inhibitory action of this compound was selective and reversible. It shows a mixed-type inhibition toward recombinant human AChE activity. Ki1 (competitive inhibition) and Ki2 values (noncompetitive inhibition) of this chemical were 610 nM and 2.7 μM, respectively. IC50 values for AChE of this compound is 3 μM. IC50 value for BuChE of this compound is >1.0 × 10−3 M. This compound inhibited AChE selectively, and the ratio of the IC50 value for BuChE to AChE was more than 330. This chemical has no affinity for α2-adrenoceptor, dopamine D2S receptor, or serotonin 5-HT4 receptors, including 5-HT4c, 5-HT4d, or 5-HT4e receptors. In in vitro studies, this compound enhanced acetylcholine- but not carbachol-induced contractile responses of guinea pig gastric antrum strips.
|
| In vivo |
Acotiamide stimulates gastric motility and improves gastric motility dysfunction in rats by inhibiting AChE activity. It improves stress-induced delayed gastric emptying in rats. This compound also enhances gastric motility by inhibiting AChE activity without affecting QT interval in dogs.
|
References |
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































