research use only
Amiloride HCl Sodium Channel inhibitor
Cat.No.S1811
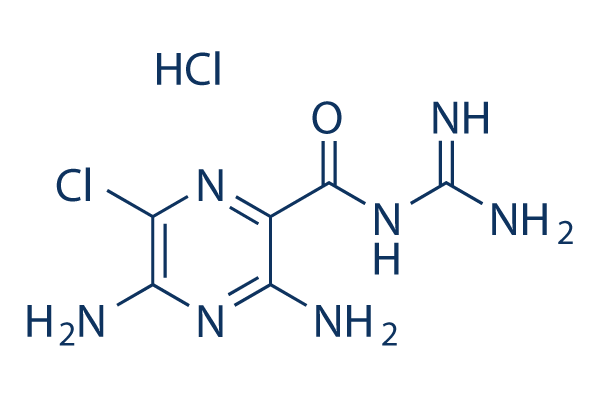
Chemical Structure
Molecular Weight: 266.09
Quality Control
| Related Targets | CFTR CRM1 CD markers AChR Calcium Channel Potassium Channel GABA Receptor TRP Channel ATPase GluR |
|---|---|
| Other Sodium Channel Inhibitors | Camostat Mesilate A-803467 cariporide Veratramine Tolperisone HCl Bulleyaconi cine A Vinpocetine Tenapanor PF-06869206 Sparteine |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| D17 | Cell viability assay | 72 h | IC50=110.66 μM | 30556178 | ||
| Abrams | Cell viability assay | 72 h | IC50=121.61 μM | 30556178 | ||
| Dharma | Cell viability assay | 72 h | IC50=148.37 μM | 30556178 | ||
| YD-10B | Function assay | 4 mM | 4 h | amiloride strongly blocked the meridianin C‐induced accumulation of vacuoles in YD-10B cells | 30246484 | |
| NS20Y | Function assay | Amiloride dose dependently inhibits the ASIC current in NS20Y cells with an IC50 of 11.04 μM | 27342076 | |||
| COS-7 | Function assay | Binding affinity for HA-tagged mutant human Adenosine A2A receptor (H250N) using [3H]-CGS-21,680 as radioligand expressed in COS-7 cells, Ki=3.28μM | 9258366 | |||
| COS-7 | Function assay | Binding affinity for HA-tagged mutant human Adenosine A2A receptor (V84L), using [3H]CGS-21680 as radioligand expressed in COS-7 cells, Ki=11.6μM | 9258366 | |||
| COS-7 | Function assay | Binding affinity for HA-tagged wild type human Adenosine A2A receptor (WT) using [3H]CGS-21680 as radioligand expressed in COS-7 cells, Ki=12μM | 9258366 | |||
| MDCK | Function assay | TP_TRANSPORTER: inhibition of TEA uptake in OCT2-expressing MDCK cells, Ki=4.7μM | 11758759 | |||
| MDCK | Function assay | TP_TRANSPORTER: inhibition of TEA uptake in OCT1-expressing MDCK cells, Ki=6.9μM | 11758759 | |||
| AP1 | Function assay | Inhibition of Amphiuma tridactylum NHE1 mutant containing TM10-12 domain of Pleuronectes americanus expressed in chinese hamster AP1 cells assessed as intracellular pHi recovery by ammonium chloride prepulse protocol, IC50=38μM | 17493937 | |||
| AP1 | Function assay | Inhibition of Pleuronectes americanus NHE1 C-terminal mutant containing TM7 domain of Amphiuma tridactylum expressed in chinese hamster AP1 cells assessed as intracellular pHi recovery by ammonium chloride prepulse protocol | 17493937 | |||
| AP1 | Function assay | Inhibition of Pleuronectes americanus NHE1 TM4 mutant containing TFFLF sequence of Amphiuma tridactylum expressed in chinese hamster AP1 cells assessed as intracellular pHi recovery by ammonium chloride prepulse protocol | 17493937 | |||
| HBE | Function assay | Inhibition of human ENaC in HBE cells by short-circuit current technique, IC50=0.22μM | 22197144 | |||
| FRT | Function assay | Inhibition of guinea pig ENaCbeta1/gamma1 expressed in FRT cells by short-circuit current technique, IC50=0.54μM | 22197144 | |||
| HBE | Function assay | Blockade of human ENaC expressed in HBE cells by short-circuit current assay, IC50=0.22μM | 22425452 | |||
| FRT | Function assay | Blockade of guinea pig ENaC expressed in FRT cells by short-circuit current assay, IC50=0.54μM | 22425452 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for A673 cells) | 29435139 | |||
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for U-2 OS cells | 29435139 | |||
| AP1 | Function assay | Inhibition of rat NHE1 expressed in chinese hamster AP1 cells assessed as inhibition of acid-induced 22Na+ influx by liquid scintillation spectroscopy, Ki=1μM | ChEMBL | |||
| AP1 | Function assay | Inhibition of full length human C-terminal HA-tagged human NHE5 expressed in chinese hamster AP1 cells assessed as inhibition of acid-induced 22NA+ influx by liquid scintillation spectroscopy, Ki=21μM | ChEMBL | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 53 mg/mL
(199.18 mM)
Water : 6 mg/mL Ethanol : 5 mg/mL |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 266.09 | Formula | C6H8ClN7O.HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 2016-88-8 | -- | Storage of Stock Solutions |
|
|
| Synonyms | MK-870 HCl | Smiles | C1(=C(N=C(C(=N1)Cl)N)N)C(=O)N=C(N)N.Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
Sodium channel
T-type calcium channel
uPA
7 μM(Ki)
|
|---|---|
| In vitro |
Amiloride is a relatively selective inhibitor of the epithelial sodium channel (ENaC) with an IC50 (the concentration required to reach 50% inhibition of an ion channel) in the concentration range of 0.1 to 0.5 μM. Amiloride is a relatively poor inhibitor of the the Na+/H+ exchanger (NHE) with an IC50 as low as 3 μM in the presence of a low external [Na+] but as high as 1 mM in the presence of a high [Na+]. Amiloride is an even weaker inhibitor of the Na+/Ca2+ exchanger (NCX), with an IC50 of 1 mM. Amiloride (1 μM) and submicromolar doses of Benzamil (30 nM), doses known to inhibit the ENaC, inhibit the myogenic vasoconstriction response to increasing perfusion pressure by blocking the activity of ENaC proteins. Amiloride completely inhibits Na+ influx in doses known to be relatively specific for ENaC (1.5 μM) in vascular smooth muscle cells (VSMC). |
| In vivo |
Amiloride at 1 mg/kg/day subcutaneously is found to reverse the initial increases in collagen deposition and prevent any further increases in the DOCA-salt hypertensive rat. Amiloride delays the onset of proteinuria and improved brain and kidney histologic scores in the saline-drinking, stroke-prone spontaneously hypertensive rats (SHRSP). compared with controls. Amiloride antagonizes or prevents actions of aldosterone in these cells and in cardiovascular and renal tissues in animals with salt-dependent forms of hypertension. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
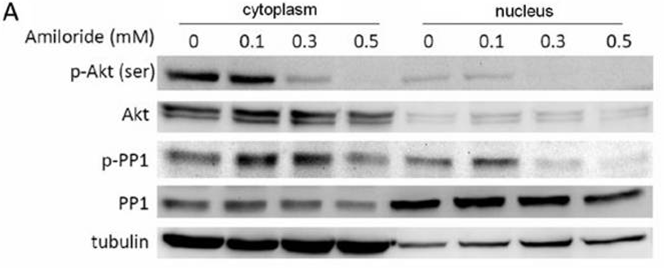
| Western blot | p-AKT / AKT / p-PP1 / PP1 |
|
21694768 |
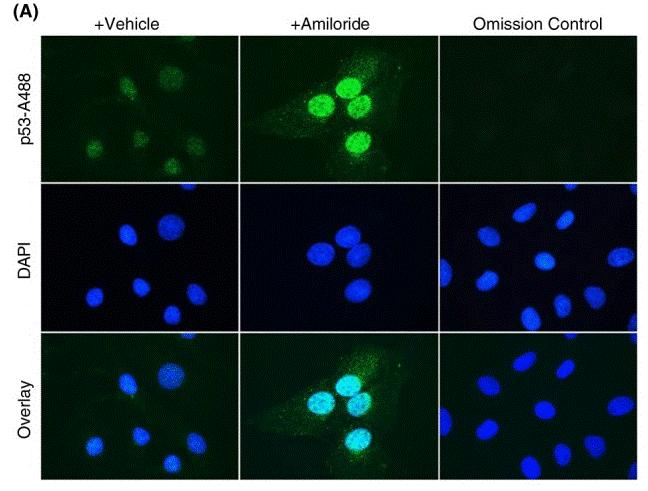
| Immunofluorescence | p53 |
|
30556178 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05044611 | Recruiting | Bipolar Disorder |
Assistance Publique - Hôpitaux de Paris |
January 11 2023 | Phase 4 |
| NCT04181008 | Completed | Pharmacokinetics |
University of Utah|Center for Addiction and Mental Health |
September 28 2020 | Early Phase 1 |
| NCT02323100 | Terminated | Cystic Fibrosis |
National Jewish Health|University of Alabama at Birmingham|Children''s Hospital of Philadelphia|Johns Hopkins University|Horizon Pharma Ireland Ltd. Dublin Ireland |
December 2 2018 | Phase 1|Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































