research use only
EGCG ((-)-Epigallocatechin Gallate) Telomerase inhibitor
Cat.No.S2250
-Epigallocatechin-gallate-chemical-structure-S2250.gif)
Chemical Structure
Molecular Weight: 458.37
Quality Control
| Related Targets | HDAC PARP ATM/ATR DNA-PK WRN DNA/RNA Synthesis Topoisomerase PPAR Sirtuin Casein Kinase |
|---|---|
| Other Telomerase Inhibitors | BIBR 1532 Costunolide RHPS 4 methosulfate Cycloastragenol TAC (TERT Activator-1) Cyclogalegenol Braco-19 trihydrochloride L-778123 hydrochloride Epitalon MST-312 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| SH-SY5Y cells | Function assay | Neuroprotection against beta-amyloid peptide 1-42-induced toxicity in human SH-SY5Y cells assessed as lactate dehydrogenase release, EC50=0.03987 μM | ||||
| human MDA435/LCC6MDR cells | Function assay | 10 μM | 5 days | Modulation of P-gp (unknown origin) transfected in human MDA435/LCC6MDR cells assessed as reversible of paclitaxel resistance measured as IC50 for paclitaxel at 10 uM after 5 days by CellTiter 96 Aqueous assay, IC50=0.1226 μM | ||
| Sf9 cells | Function assay | Inhibition of His6-tagged human recombinant DNMT1 expressed in insect Sf9 cells assessed as reduction in DNA methyltransferase activity using 5'-biotinylated 45-bp unmethylated or hemimethylated oligonucleotide substrates and [3H]-AdoMet by liquid scintillation counting method, IC50=0.5 μM | ||||
| human U937 cells | Function assay | Inhibition of telomerase in human U937 cells, IC50=1 μM | ||||
| human HeLa cells | Function assay | Inhibition of telomerase in human HeLa cells using 5'-AAT CCG TCG AGC AGA GTT-3' as substrate incubated for 15 mins prior to extension reaction followed by compound washout by spin-telomeric repeat amplification protocol, IC50=1.08 μM | ||||
| CHO cells | Cytotoxic assay | 48 h | Cytotoxicity against CHO cells expressing OATP1B3 haplotype 1 after 48 hrs by fluorescence based CellTiter-Glo assay, IC50=3.2 μM | |||
| MDCK cells | Function assay | 4 days | Antiviral activity against influenza A virus (A/swine/OH/511445/2007(H1N1)) Oh7 infected in MDCK cells assessed as inhibition of viral replication after 4 days by quantitative RT-PCR, ED50=8.3 μM | |||
| human HL60 cells | Proliferation assay | 3 days | Antiproliferative activity against human HL60 cells after 3 days, IC50=9.4 μM | |||
| HSC-T6 cells | Function assay | 48 h | Antifibrotic activity against rat HSC-T6 cells assessed as inhibition of proliferation after 48 hrs by BrdU incorporation assay, IC50=9.9 μM | |||
| mouse 3T3-L1 cells | Function assay | Inhibition of G6PD-mediated NADPH production in mouse 3T3-L1 cells, IC50=25 μM | ||||
| mouse RAW264.7 cells | Function assay | 5 days | Inhibition of RANKL-induced osteoclastogenesis in mouse RAW264.7 cells assessed as decrease in TRAP-positive multi-nucleated cells after 5 days, IC50=29.8 μM | |||
| HSC-T6 cells | Proliferation assay | Antiproliferative activity against rat HSC-T6 cells assessed as reduction in cell viability, IC50=29.8 μM | ||||
| human A431 cells | Proliferation assay | 48 h | Antiproliferative activity against human A431 cells overexpressing ErbB in serum-free medium assessed as cell viability after 48 hrs by WST-1 assay, EC50=38 μM | |||
| human MDA-MB-231 cells | Proliferation assay | 24 h | Antiproliferative activity against human MDA-MB-231 cells after 24 hrs by MTT assay | |||
| human HepG2 cells | Function assay | 24 h | Inhibition of oleic acid-induced triglyceride over-accumulation in human HepG2 cells incubated for 24 hrs relative to untreated control | |||
| human HepG2 cells | Function assay | 24 h | Antioxidant activity in human HepG2 cells assessed as reduction of oleic acid-induced ROS generation incubated for 24 hrs by DHCF-DA based fluorimetric assay relative to untreated control | |||
| human Caco-2 cells | Growth inhibition assay | 25 μM | 6 days | Growth inhibition of human Caco-2 cells at 25 uM after 6 days | ||
| mouse 3T3 cells | Function assay | 1-20 μM | 12 h | Antimigratory activity against Swiss albino mouse 3T3 cells assessed as increase of cell numbers at 1 to 20 uM after 12 hrs by by scratch-wound assay | ||
| human MDA-MB-231 cells | Proliferation assay | 24 h | Antiproliferative activity against human MDA-MB-231 cells after 24 hrs by MTT assay | |||
| human Jurkat cells | Function assay | 10-55 μM | 24 h | Inhibition of TNFalpha-induced NF-kappaB activation in human Jurkat cells at 10 to 55 uM after 24 hrs by electrophoretic mobility shift assay | ||
| human K562 cells | Function assay | 24 h | Induction of 67 kDa laminin receptor expression in human K562 cells after 24 hrs by flow cytometry analysis | |||
| human HL60 cells | Function assay | 24 h | Induction of 67 kDa laminin receptor expression in human HL60 cells after 24 hrs by flow cytometry analysis | |||
| human Raji cells | Growth inhibition assay | 10 μM | 48 h | Growth inhibition against human Raji cells assessed as cell viability at 10 uM after 48 hrs by trypan blue based microscopic analysis in presence of superoxide dismutase | ||
| human PC3 cells | Proliferation assay | 10-100 μM | 48 h | Antiproliferative activity against human PC3 cells at 10 to 100 uM after 48 hrs by hemocytometric cell counting method | ||
| human A431 cells | Function assay | 100 μM | 12 h | Reduction of clustering of GFP-GPI in lipid rafts of human A431 cells at 100 uM after 12 hrs by confocal microscopic analysis | ||
| human SKBR3 cells | Function assay | 200μM | 30 mins | Downregulation of ErbB2 protein expression in human SKBR3 cells in serum free medium at 200 uM after 30 mins by immunofluorescence staining-based confocal microscopic analysis | ||
| human 293T cells | Function assay | 50 μM | 12 h | Inhibition of DYRK1A in human 293T cells assessed as reduction of GLI1 transcription activity at 50 uM after 12 hrs by dual-luciferase reporter gene assay | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 91 mg/mL
(198.52 mM)
Water : 91 mg/mL Ethanol : 91 mg/mL |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 458.37 | Formula | C22H18O11 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 989-51-5 | Download SDF | Storage of Stock Solutions |
|
|
Mechanism of Action
| Targets/IC50/Ki |
telomerase
DNMT
HER2
EGFR
FASN
|
|---|---|
| In vitro |
(-)-Epigallocatechin gallate functions as a powerful antioxidant, preventing oxidative damage in healthy cells, but also as an antiangiogenic and antitumor agent and as a modulator of tumor cell response to chemotherapy. (-)-Epigallocatechin gallate shows multiple anticancer effects, such as anti-proliferation, anti-angiogenesis, transformation prevention of various cancer cells, cancer cell cycle arrest and inhibition of tumor metastasis. (-)-Epigallocatechin gallate exerts multi-anticancer effects through regulating several cancer-related cell signal pathways (regulated the function or the expression of key signal proteins, such as nuclear factor-κB, MAPKs and activator protein-1, EGFR, IGF, COX-2 signaling pathway, and so on.), effecting methylation of cancer genes and combination of ligand with membrane receptors. (-)-Epigallocatechin gallate also shows an immunomodulating effects. Several types of immune cells in both the innate and adaptive immune systems are known to be affected in varying degrees by (-)-Epigallocatechin gallate. Among them, the dramatic effect on T cell functions has been repeatedly demonstrated, including T cell activation, proliferation, differentiation, and production of cytokines. Studies using animal models of autoimmune diseases have reported disease improvement in animals treated with green tea/EGCG. (-)-Epigallocatechin gallate displays anti-infective properties. Antiviral activities of (-)-Epigallocatechin gallate with different modes of action have been demonstrated on diverse families of viruses, such as Retroviridae, Orthomyxoviridae and Flaviviridae and include important human pathogens like human immunodeficiency virus, influenza A virus and the hepatitis C virus. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | β-catenin / p-AKT / Cyclin D1 p-MAPK / MAPK Notch1 / Notch2 |
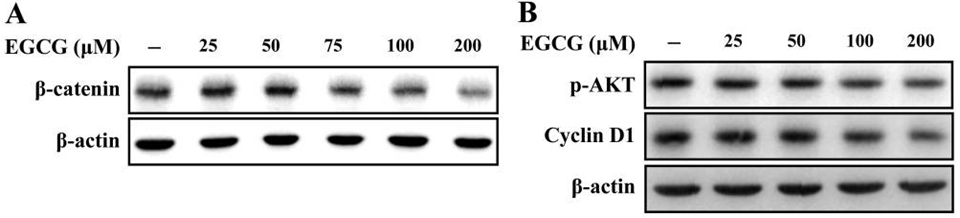
|
28693189 |
| Growth inhibiton assay | Cell proliferation Cell viability |
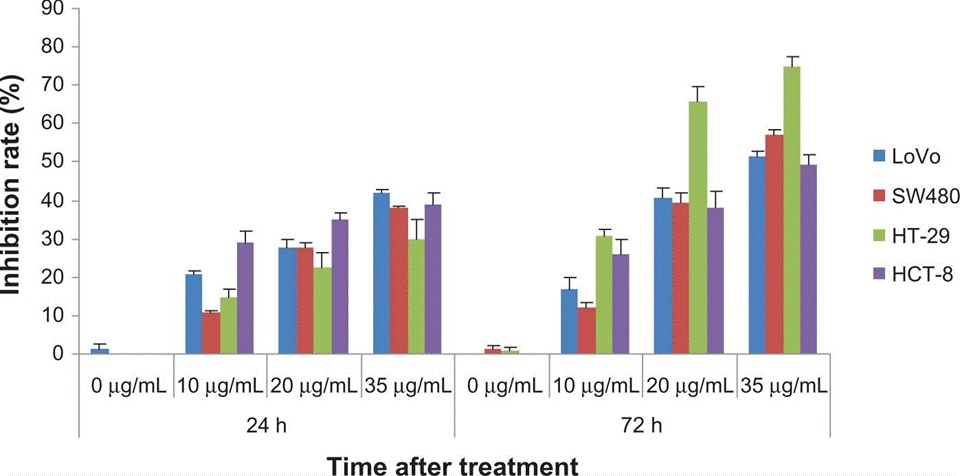
|
23525843 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05758571 | Recruiting | Interstitial Pneumonia|Neoplasms Malignant |
Shandong Cancer Hospital and Institute |
January 5 2023 | Phase 1|Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































