research use only
GS-5734 (Remdesivir) Antiviral Agent
Cat.No.S8932
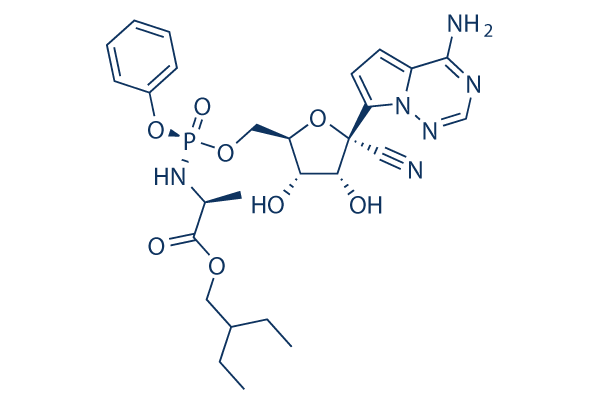
Chemical Structure
Molecular Weight: 602.58
Quality Control
| Related Targets | Integrase Bacterial Antibiotics Anti-infection Fungal COVID-19 Parasite Reverse Transcriptase HIV HCV Protease |
|---|---|
| Other Antiviral Inhibitors | Moroxydine HCl Aloperine GS-441524 Harringtonine Oleanolic Acid NGI-1 U18666A Aloin B LL-37 acetate Lapachol |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| Hep2 | Antiviral activity assay | 4 days | EC50 = 0.015 μM | 28124907 | ||
| TERT-immortalized HMVEC | Antiviral activity assay | 3 to 4 days | EC50 = 0.053 μM | 28124907 | ||
| HuH7 | Antiviral activity assay | 3 days | EC50 = 0.057 μM | 28124907 | ||
| HuH7 | Antiviral activity assay | 3 days | EC50 = 0.07 μM | 28792763 | ||
| macrophages | Antiviral activity assay | 48 h | EC50 = 0.086 μM | 28124907 | ||
| macrophages | Antiviral activity assay | 48 h | EC50 = 0.086 μM | 28792763 | ||
| HeLa | Antiviral activity assay | 48 h | EC50 = 0.1 μM | 28124907 | ||
| HeLa | Antiviral activity assay | 48 h | EC50 = 0.14 μM | 28792763 | ||
| MT4 | Cytotoxicity assay | 4 to 5 days | CC50 = 1.7 μM | 28124907 | ||
| Hep2 | Cytotoxicity assay | 4 to 5 days | CC50 = 6.1 μM | 28124907 | ||
| HuH7 | Cytotoxicity assay | 3 days | CC50 = 36 μM | 28124907 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(165.95 mM)
Ethanol : 16 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 602.58 | Formula | C27H35N6O8P |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 1809249-37-3 | -- | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CCC(CC)COC(=O)C(C)NP(=O)(OCC1C(C(C(O1)(C#N)C2=CC=C3N2N=CN=C3N)O)O)OC4=CC=CC=C4 | ||
Mechanism of Action
| In vitro |
Remdesivir (GS-5734) exhibits antiviral activity against multiple variants of EBOV in cell-based assays (EC50=0.06-0.14 μM) and broad-spectrum antiviral activity in vitro against other pathogenic RNA viruses. This compound acts as a broad-spectrum therapeutic to protect against CoVs with EC50 of 0.03 μM for murine hepatitis virus in delayed brain tumor cells and 0.074 μM for SARS-CoV and MERS-CoV in HAE cells. |
|---|---|
| In vivo |
Regardless of the time of initiation, treatment with Remdesivir (GS-5734) at 3 mg/kg confers improved survival. All animals in which a 10 mg/kg regimen is initiated 3 days after virus exposure survive to the end of the in-life phase. However, the antiviral effects are consistently greater in animals administered repeated 10 mg/kg doses. The 10 mg/kg D3 (administered beginning 3 days after virus exposure) regimen is associated with amelioration of EVD-related clinical disease signs and markers of coagulopathy and end organ pathophysiology. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | delayed chain termination of RNA synthesis tGFP-BlaR / Actin ACE2 / Actin VP0 / VP2 |
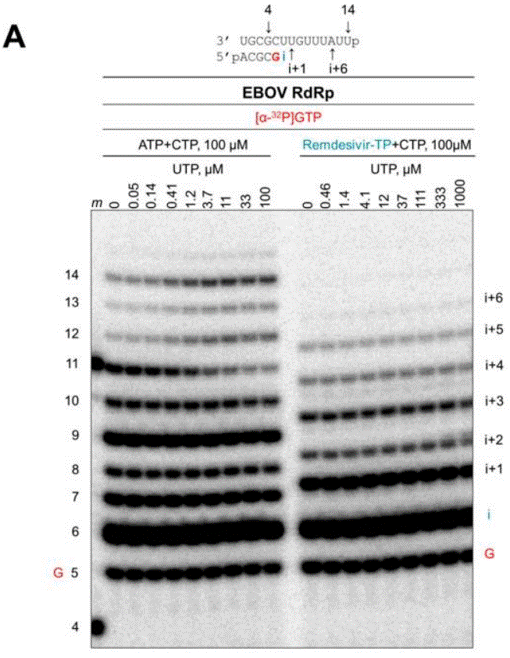
|
30987343 |
| Growth inhibition assay | Cell viability Cell viability Cell viability |
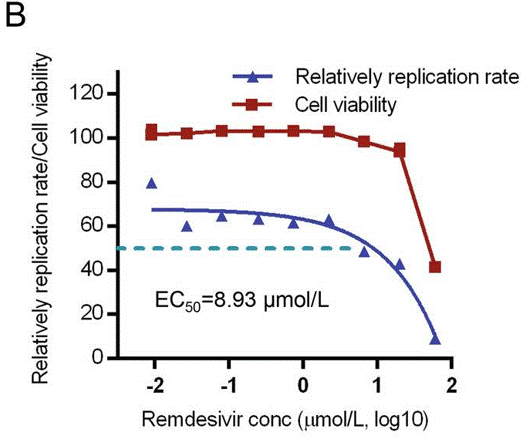
|
33835389 |
| Pharmacokinetics | plasma concentration plasma concentration |
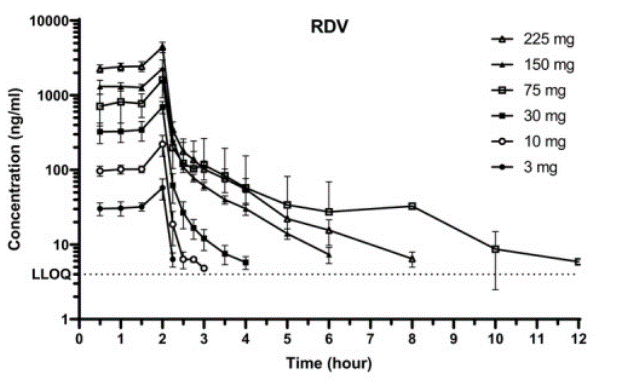
|
32589775 |
| Immunofluorescence | MERS-CoV S Protein viral S protein and E-caherin SARS-CoV-2 / E‐cadherin / VE‐cadherin SARS-CoV-2 viral dsRNA replication intermediates |
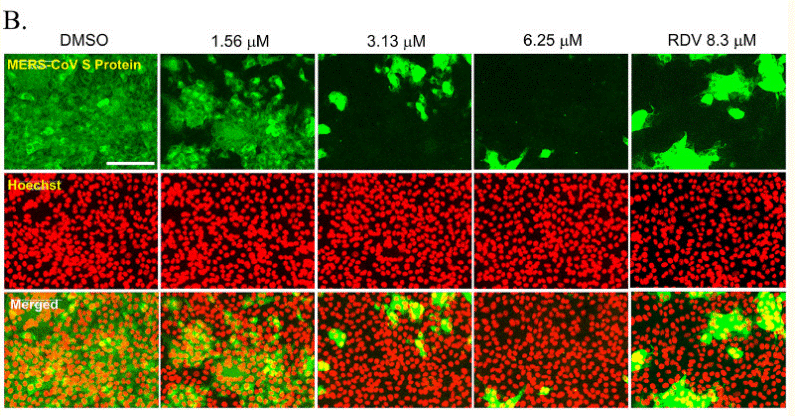
|
33376043 |
| Viral loads and virus titers | SARS-CoV-2 |
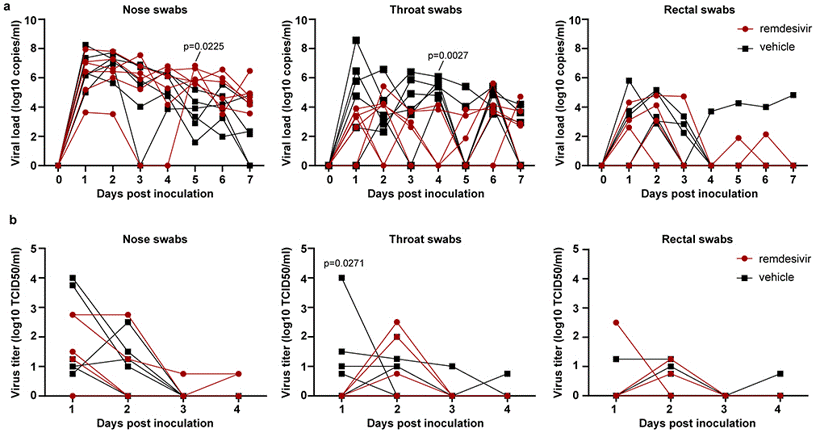
|
32516797 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































