research use only
GS-441524 Antiviral inhibitor
Cat.No.S6814
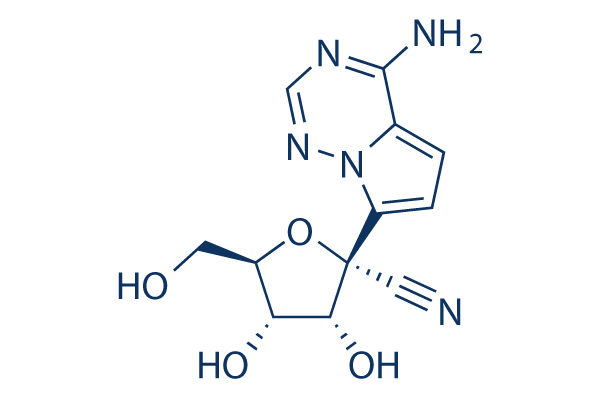
Chemical Structure
Molecular Weight: 291.26
Quality Control
| Related Targets | Integrase Bacterial Antibiotics Anti-infection Fungal COVID-19 Parasite Reverse Transcriptase HIV HCV Protease |
|---|---|
| Other Antiviral Inhibitors | Moroxydine HCl Aloperine Harringtonine Oleanolic Acid NGI-1 U18666A Aloin B Lapachol LL-37 acetate Saikosaponin B2 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| HuH7 | Function assay | 10 uM | 24 hrs | Cmax in human HuH7 cells assessed as triphosphate concentration per million cells at 10 uM incubated for 24 hrs measured after washout by LC-MS analysis, Cmax=0.0000176μM | 22446091 | |
| Vero | Antiviral assay | 24 hrs | Antiviral activity against Parainfluenza 3 C 243 infected in african green monkey Vero cells assessed as inhibition of virus induced cytopathic effect after 24 hrs by MTS assay, EC50=1.71μM | 22446091 | ||
| Vero | Antiviral assay | 24 hrs | Antiviral activity against SARS coronavirus Toronto-2 infected in african green monkey Vero cells assessed as inhibition of virus induced cytopathic effect after 24 hrs by MTS assay, EC50=2.24μM | 22446091 | ||
| HuH7 | Antiviral assay | 24 hrs | Antiviral activity against Hepatitis C virus genotype 1b infected in human HuH7 cells assessed as inhibition of virus induced cytopathic effect after 24 hrs by MTS assay, EC50=4.1μM | 22446091 | ||
| Vero E6 | Antiviral assay | 24 hrs | Antiviral activity against Dengue virus type 2 New Guinea C infected in african green monkey Vero E6 cells assessed as inhibition of virus induced cytopathic effect after 24 hrs by MTS assay, EC50=9.46μM | 22446091 | ||
| HeLa | Antiviral assay | 24 hrs | Antiviral activity against Yellow fever virus 17D infected in human HeLa cells assessed as inhibition of virus induced cytopathic effect after 24 hrs by MTS assay, EC50=11μM | 22446091 | ||
| MDCK | Antiviral assay | 24 hrs | Antiviral activity against Influenza A virus H3N2 infected in MDCK cells assessed as inhibition of virus induced cytopathic effect after 24 hrs by MTS assay, EC50=27.9μM | 22446091 | ||
| Hep2 | Antiviral assay | 4 days | Antiviral activity against Respiratory syncytial virus A2 infected in human Hep2 cells assessed as reduction of virus-induced cytopathic effect after 4 days by Cell-Titer Glo assay, EC50=0.53μM | 28124907 | ||
| HMVEC | Antiviral assay | 3 to 4 days | Antiviral activity against GFP-fused Ebolavirus infected in TERT-immortalized HMVEC assessed as reduction in viral replication preincubated with cells followed by viral infection measured after 3 to 4 days by fluorescence assay, EC50=0.78μM | 28124907 | ||
| HuH7 | Antiviral assay | 3 days | Antiviral activity against Hepatitis C virus genotype 1b infected in human HuH7 cells preincubated with cells for 3 days followed by viral infection by luciferase assay, EC50=4.1μM | 28124907 | ||
| HuH7 | Antiviral assay | 3 days | Antiviral activity against Ebolavirus infected in human HuH7 cells after 3 days by end point dilution assay, EC50=1.5μM | 28792763 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 58 mg/mL
(199.13 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 291.26 | Formula | C12H13N5O4 |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 1191237-69-0 | -- | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | C1=C2C(=NC=NN2C(=C1)C3(C(C(C(O3)CO)O)O)C#N)N | ||
Mechanism of Action
| Targets/IC50/Ki |
FIPV
(Cell-free assay) 0.78 μM(EC50)
|
References |
|---|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05996744 | Terminated | COVID-19 |
Gilead Sciences |
December 26 2023 | Phase 2|Phase 3 |
| NCT05715528 | Completed | COVID-19 |
Gilead Sciences |
February 8 2023 | Phase 3 |
| NCT05603143 | Terminated | COVID-19 |
Gilead Sciences |
November 5 2022 | Phase 3 |
| NCT04582266 | Completed | COVID-19 |
National Institute of Allergy and Infectious Diseases (NIAID)|Gilead Sciences |
March 31 2021 | -- |
| NCT04859244 | Completed | COVID-19 |
Copycat Sciences LLC |
January 1 2021 | Phase 1 |
| NCT04539262 | Completed | COVID-19 |
Gilead Sciences |
September 14 2020 | Phase 1|Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































