research use only
BI-10773 (Empagliflozin) SGLT2 inhibitor
Cat.No.S8022
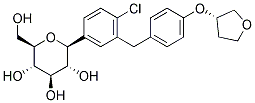
Chemical Structure
Molecular Weight: 450.91
Quality Control
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| HEK293 | Function assay | 15 mins | Inhibition of recombinant human SGLT2 expressed in HEK293 cells assessed as decrease in [14C]-AMG uptake preincubated for 15 mins followed by [14C]-AMG addition and measured after 4 hrs by Topcount method, IC50 = 0.0031 μM. | ChEMBL | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 90 mg/mL
(199.59 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 450.91 | Formula | C23H27ClO7 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 864070-44-0 | -- | Storage of Stock Solutions |
|
|
| Synonyms | BI 10773 | Smiles | C1COCC1OC2=CC=C(C=C2)CC3=C(C=CC(=C3)C4C(C(C(C(O4)CO)O)O)O)Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
SGLT2
3.1 nM
|
|---|---|
| In vitro |
Empagliflozin shows >2500-fold selectivity for hSGLT-2 over hSGLT-1 (IC50 8300 nM) and >3500-fold selectivity over hSGLT-4, it exhibits >350-fold selectivity over hSGLT-5 (IC50=1100 nM) and >600-fold selectivity over hSGLT-6. No relevant inhibition of GLUT1 is observed up to 10 μM of this compound. In kinetic binding experiments, [3H]-empagliflozin displays a high affinity for SGLT-2 with a mean Kd of 57 nM in the absence of glucose, and shows a half-life of [3H]-empagliflozin-binding to SGLT-2 of 59 min in the absence of glucose. Its binding to SGLT-2 is competitive with glucose.
|
| Kinase Assay |
[14C]-monosaccharide uptake inhibition experiments
|
|
Stable cell lines over-expressing hSGLT-1, -2, -4, -5 or -6 or rSGLT-1 or -2 are used for the sodium-dependent monosaccharide transport inhibition assay. Cells are pre-incubated in 200 μL uptake buffer (10 mM HEPES, 137 mM NaCl, 5.4 mM KCl, 2.8 mM CaCl2, 1.2 mM MgCl2, 50 μg/ml Gentamycin, 0.1% BSA) for 25 minutes at 37°C. 10 μM Cytochalasin B and this compound is added at different concentrations 15 minutes before the initiation of the uptake experiment. The uptake reaction is started by the addition of 0.6 μCi [14C]-labelled monosaccharide i.e. [14C]-labelled AMG, glucose, fructose, mannose or myo-inositol, in 0.1 mM AMG (or the respective non-radioactive monosaccharide). After incubation for 60 minutes (hSGLT-5), 90 minutes (hSGLT-4) or 4 hours (hSGLT-2) at 37°C, the cells are washed three times with 300 μL PBS and then lysed in 0.1 N NaOH with intermittent shaking for 5 minutes. The lysate is mixed with 200 μL MicroScint 40 and shaken for 15 minutes and counted for radioactivity in the TopCount NXT. For SGLT-4 and SGLT-5 assays cells are pre-incubated in pre-treatment buffer (uptake buffer containing choline chloride instead of NaCl) for 25 minutes prior to addition of uptake buffer.
|
|
| In vivo |
High exposure of empagliflozin is achieved in dogs, with plasma concentrations >100-fold above IC50 measured 24 h after administration of 5 mg/kg this compound. The total plasma clearance of this chemical in ZDF rat is 43 mL/min/kg, while in dogs is lower at 1.8 mL/min/kg. Cmax of this agent in ZDF rat and dogs is 167 nM and 17254 nM, respectively. Terminal elimination half-life in ZDF rat and dogs is 1.5 h and 6.3 h, respectively. Bioavailability of this drug in ZDF rat is 33.2%, while in dogs is higher at 89.0%. Long-term treatment with this medication, improves glycaemic control
and features of metabolic syndrome in diabetic rats.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06012266 | Not yet recruiting | Heart Failure |
Centre Hospitalier Universitaire Vaudois|Great Ormond Street Hospital for Children NHS Foundation Trust|University College London |
August 2024 | Phase 2 |
| NCT06308679 | Not yet recruiting | Healthy Vollunteer |
Pharma Nueva |
May 28 2024 | Phase 1 |
| NCT06339788 | Not yet recruiting | Healthy Volunteers |
Handok Inc. |
April 2024 | Phase 1 |
| NCT06013865 | Recruiting | Kidney Transplant|Type 2 Diabetes |
VA Office of Research and Development|Iowa City VA Health Care System|VA Pittsburgh Healthcare System|Nashville VA Medical Center |
April 5 2024 | Phase 4 |
| NCT06021145 | Recruiting | Type 1 Diabetes |
McGill University Health Centre/Research Institute of the McGill University Health Centre|Diabetes Canada |
March 2024 | Phase 4 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































