research use only
Dofetilide Potassium Channel inhibitor
Cat.No.S1658
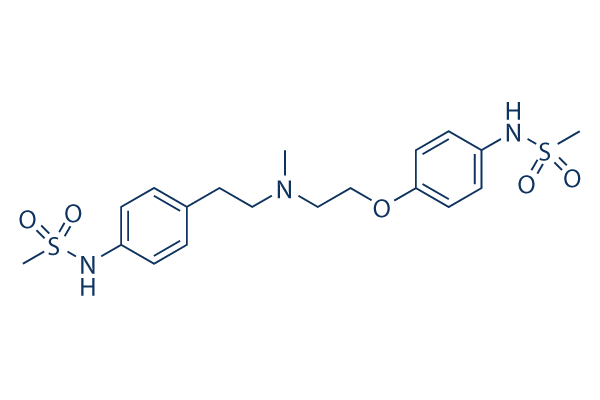
Chemical Structure
Molecular Weight: 441.56
Quality Control
| Related Targets | CFTR CRM1 CD markers AChR Calcium Channel Sodium Channel GABA Receptor TRP Channel ATPase GluR |
|---|---|
| Other Potassium Channel Inhibitors | Nicorandil TRAM-34 Nigericin Sophocarpine ML133 HCl Hydralazine HCl Gliquidone PAP-1 E-4031 dihydrochloride Ajmaline |
Solubility
|
In vitro |
DMSO
: 88 mg/mL
(199.29 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 441.56 | Formula | C19H27N3O5S2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 115256-11-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | UK-68798 | Smiles | CN(CCC1=CC=C(C=C1)NS(=O)(=O)C)CCOC2=CC=C(C=C2)NS(=O)(=O)C | ||
Mechanism of Action
| Targets/IC50/Ki |
Potassium channel
|
|---|---|
| In vitro |
Dofetilide blocks HERG currents in excised macro patches of Xenopus oocytes. This compound (1 μM) reduces the amplitude of IKr to 61% of control currents in guinea pig cardiomyocytes, as measured by 200-ms test pulses and analysis of the deactivating tail currents of IKr. It increases apico-basal disparity of repolarization, due to a more marked increase of ERPs in the apex than in the base in the intact canine heart.
|
| In vivo |
Dofetilide (100 mg/kg, i.v.) does not suppress automaticity arrhythmias induced by two-stage coronary ligation and epinephrine or the coronary ligation and reperfusion arrhythmias, but suppresses the reentry arrhythmia induced by PES in dogs with old myocardial infarction (MI). This compound also shows antiarrhythmic effect in some dogs with digitalis arrhythmia. It increases QT interval and shows negative chronotropic effect like that of other class III drugs, but is different in antiarrhythmic profiles from those of other class III agents such as D-sotalol, E-4031, and MS-551 in that it does not prevent the occurrence of ventricular fibrillation (VF) immediately after coronary reperfusion and has some antiarrhythmic effects on digitalis arrhythmia. This chemical causes increased resorptions and the same stage-dependent malformations in Sprague-Dawley rats.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05906732 | Recruiting | Long QT Syndrome |
Thryv Therapeutics Inc. |
March 12 2023 | Phase 1|Phase 2 |
| NCT01097330 | Terminated | Defibrillators Implantable|Tachycardia Ventricular |
Population Health Research Institute|Abbott Medical Devices|Hamilton Health Sciences Corporation |
August 2010 | Phase 3 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































