research use only
Hydralazine HCl Potassium Channel activator
Cat.No.S2562
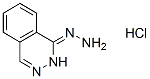
Chemical Structure
Molecular Weight: 196.64
Quality Control
| Related Targets | CFTR CRM1 CD markers AChR Calcium Channel Sodium Channel GABA Receptor TRP Channel ATPase GluR |
|---|---|
| Other Potassium Channel Inhibitors | Nicorandil TRAM-34 Nigericin Sophocarpine ML133 HCl Gliquidone PAP-1 E-4031 dihydrochloride Ajmaline NS8593 Hydrochloride |
Solubility
|
In vitro |
Water : 39 mg/mL
DMSO
: Insoluble
Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 196.64 | Formula | C8H8N4.HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 304-20-1 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | C1=CC=C2C(=C1)C=NN=C2NN.Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
Potassium Channel
|
|---|---|
| In vitro |
Hydralazine impairs up-regulation of RAG-2 gene expression and reduces secondary Ig gene rearrangements. Hydralazine subverts B lymphocyte tolerance to self and contributes to generation of pathogenic autoreactivity by disrupting receptor editing. Hydralazine directly scavenges free acrolein, decreasing intracellular acrolein availability and thereby suppressing macromolecular adduction. Hydralazine inhibits cross-linking if adding 30 min after commencing acrolein exposure but is ineffective if added after a 90-min delay. Hydralazine (0.1-10 mM) inhibits both extracellular and intracellular ROS production by inflammatory macrophages, by a ROS-scavenging mechanism probably affecting superoxide radical (O(2)(*-))-generation by xanthine oxidase (XO) and nicotinamide adenine dinucleotide/nicotinamide adenine dinucleotide phosphate (NADH/NADPH) oxidase. Hydralazine (0.1-10 mM) significantly reduces NO(*) generation, and this effect is attributable to an inhibition of NOS-2 gene expression and protein synthesis. Hydralazine also effectively blocks COX-2 gene expression which perfectly correlated with a reduction of protein levels and PGE(2) synthesis. Hydralazine protects against not only acrolein-mediated injury, but also compression in guinea pig spinal cord ex vivo. Hydralazine can significantly alleviate acrolein-induced superoxide production, glutathione depletion, mitochondrial dysfunction, loss of membrane integrity, and reduces compound action potential conduction.
|
| In vivo |
Hydralazine affords strong, dose-dependent protection against the increases in plasma marker enzymes but not the hepatic glutathione depletion produced by allyl alcohol in mice.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05096728 | Completed | Preeclampsia With Severe Features |
Ohio State University |
December 1 2021 | -- |
| NCT04164004 | Active not recruiting | Heart Failure |
Stanford University |
August 30 2021 | Not Applicable |
| NCT03619317 | Unknown status | Cancer of Esophagus |
Aarhus University Hospital Skejby|Danish Cancer Society |
June 25 2018 | -- |
| NCT01587313 | Completed | Healthy |
Arbor Pharmaceuticals Inc. |
April 2012 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































